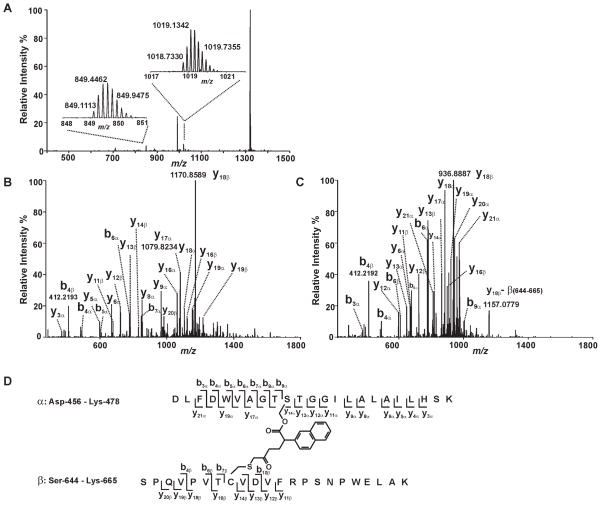
Figure 5. Mass spectrometric evidence for the cross-linking of S465 and C651.
Trypsinized iPLA2β treated with (S)-BEL was analyzed by ESI-LC/MS/MS as described in “Experimental Procedures”. A, The survey full mass spectrum acquired using the Orbitrap mass analyzer with a resolution r = 30,000 at m/z 400 shows that the cross-linked peptide is detected as ions in two different charge states. The peaks at m/z 849.1113 and 1018.7330 represent the monoisotopic ions of the +6- or +5- charged cross-linked peptide, respectively. Each of the ions were fragmented by CID in the ion trap, and the fragments were analyzed in the Orbitrap mass analyzer with a resolution r = 15,000 at m/z 400. B, the tandem mass spectrum (MS2 spectrum) of the ion at m/z 1018.7330 Th. C, the tandem mass spectrum (MS2 spectrum) of the ion at m/z 849.1113 Th. D, the structure and the identified fragments of the cross-linked peptides. The S465-containing tryptic peptide (α) and C651-containing tryptic peptide (β) are cross-linked by a link-age between S465 and C651 via a BEL hydrolytic product.