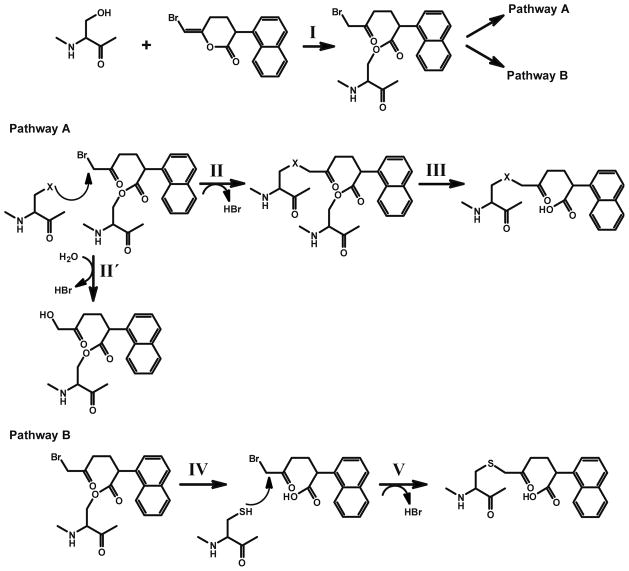
Scheme 1. Proposed mechanisms for the inactivation of the iPLA2β by BEL.
I.. Nucleophilic attack of the carbonyl carbon of the bromoenol lactone ring of BEL by the catalytic serine (S465) of iPLA2β generates the electrophilic α-bromomethyl ketone.
Pathway A:
II. The α-bromomethyl ketone remains tethered to the active site through an acyl link-age. An appropriately positioned nucleophilic residue (within the active site) then attacks the α-carbon of the ring-opened BEL intermediate to eliminate bromide resulting in the stable alkylation of the neighboring nucleophilic residue in iPLA2β. Hydroxide ion or water can also be the attacking nucleophile (II′).
III. Following deacylation of the active site serine by iPLA2β catalyzed hydrolysis, the stable α-ketone remains covalently linked to the active site of the enzyme through the adjacent nucleophilic amino acid resulting in inhibition of enzyme activity by the active site.
Pathway B:
IV. The acyl-enzyme intermediate undergoes hydrolysis to release the α-bromomethyl ketone carboxylic acid.
V. The diffusible α-bromomethyl ketone carboxylic acid reacts with cysteine residues on the enzyme, resulting in the covalent modification of the enzyme at sites distant to the active site.