Figure. Artist's rendition of the dosing scheme employed to bilaterally target the substantia nigra and putamen with AAV2-NRTN (CERE-120).
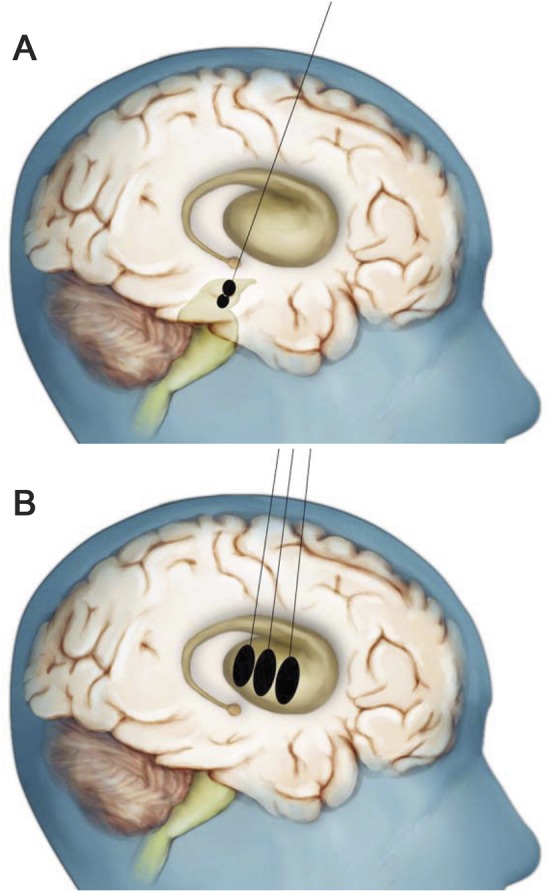
(A) A single needle tract was used to deliver 2 infusions into each substantia nigra (SN). (B) Three additional needle tracts were used to deliver 3 equally spaced infusions into each putamen. Note that only a single burr hole was required to accommodate the 4 needle tracts per each hemisphere. The initial, low-dose cohort received the same dose of CERE-120 into the SN as the high-dose cohort, but only about one-fourth the putaminal dose. See text for additional details. This figure is a variation of one previously published in “Translating the therapeutic potential of neurotrophic factors to clinical ‘proof of concept’: a personal saga achieving a career-long quest”13 and in “Advancing neurotrophic factors as treatments for age-related neurodegenerative diseases: developing and demonstrating ‘clinical proof-of-concept’ for AAV-neurturin (CERE-120) in Parkinson's disease,”1 © Elsevier (2012) with permission.
