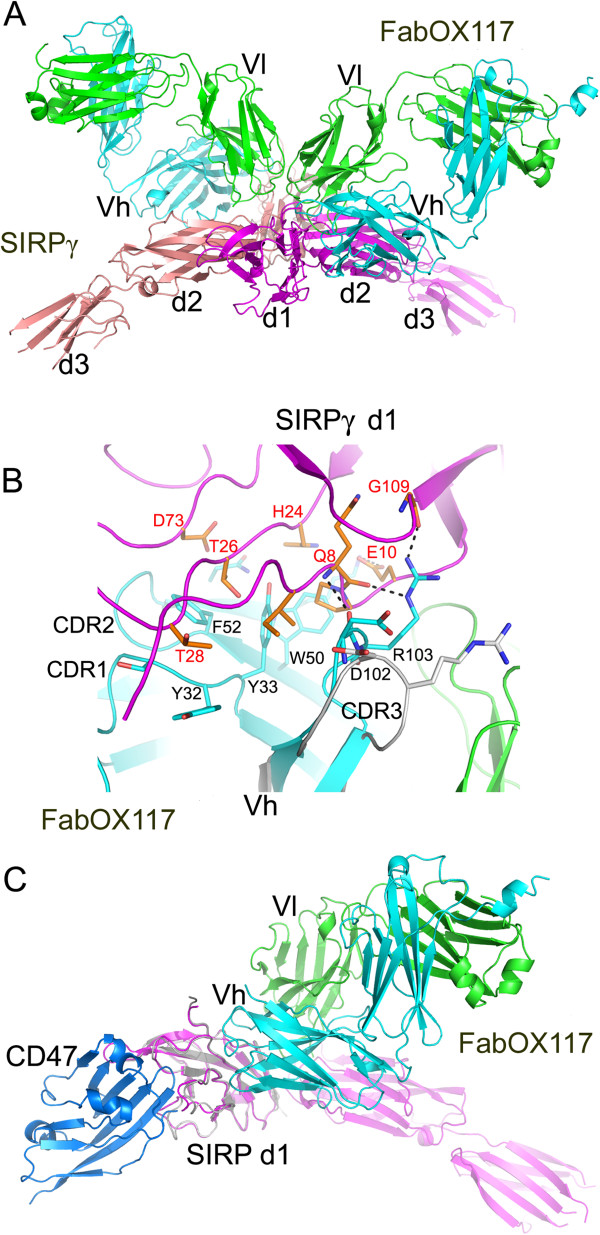
Figure 1.
Structure of the FabOX117: SIRPγ complex. (A) Overview of the FabOX117: SIRPγ complex showing two SIRPγ (coloured magenta and pink) molecules forming a dimer in the asymmetric unit each bound to a Fab fragment of the monoclonal antibody, OX117 [3], (heavy chain coloured cyan, light chain coloured green) (B) Overlay of bound/unbound (PDB id 3DIF15) FabOX117 showing the change in position of CDR3 of the heavy chain variable domain in bound (cyan) compared to the unbound (grey) FabOX117. The key residues involved in the interaction between Vh and d1 of SIRPγ (coloured orange) are shown in stick representation. (C) Comparison of SIRPγ: FabOX117 (coloured as Figure 1A) and SIRPα: CD47 complexes (coloured grey and blue respectively) showing that both CD47 and FabOX117 can bind to SIRPγ d1 at the same time.