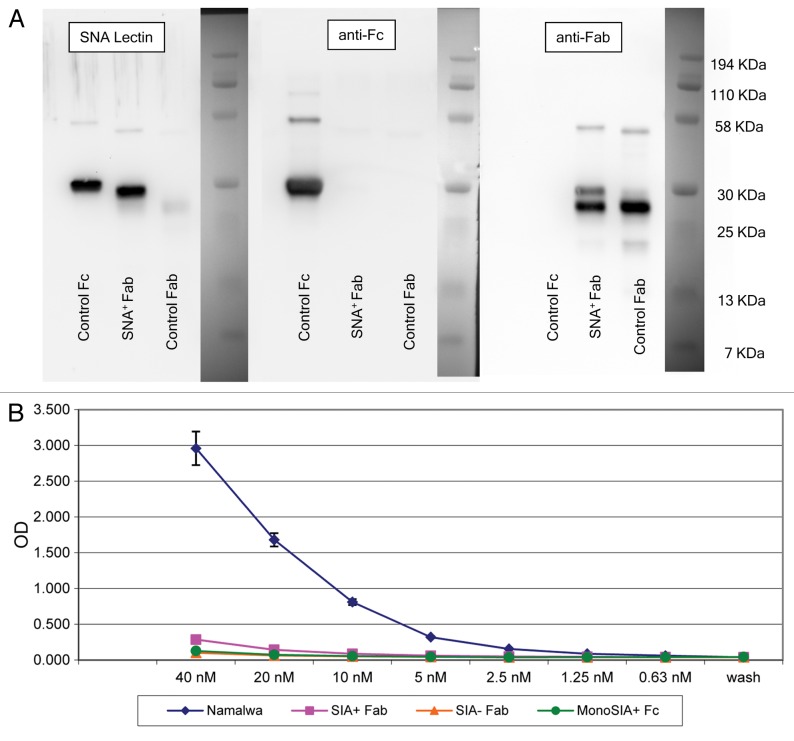
Figure 5. Sialic acid-containing (Sia+) Fab fragments do not bind soluble DC-SIGN. (A) Western and lectin blots of purified Sambucus nigra agglutinin (SNA)+ Fab fragments from human IgG. SNA+ Fab fragments were probed with the SNA lectin or antibodies specific for human Fc or Fab fragments. Control Fab and Fc fragments were obtained commercially. Only a minor fraction of control Fab fragments terminates with α2,6-Sia, as defined by lectin blotting (left panel). Conversely, the control Fc fragment contains Sia, as defined by positive signal in the lectin blot. This control Fc is likely to contain largely monosialylated glycoforms, as only a minor fraction is retarded on SNA lectin columns (data not shown), and does not bind soluble DC-SIGN (sDC-SIGN, see below). The two species identified in the Fab fragments obtained from IVIG (right panel) may represent different glycoforms of Sia+ Fabs. In support of this, the digestion of this material with sialidase results in a complete absence of either of the two species in SNA lectin blots, and peptide-N-glycosidase digests of Sia+ Fabs migrate as a single faster species when probed with anti-Fab antibodies (data not shown). The preparation is operationally free of Fc components (middle panel). (B) ELISA binding of SNA+ Fab fragments from IVIG. Diamonds, intact 4E10 positive controls; Squares, Sia+ Fabs; triangles, Sia− (asialo) Fabs; circles, monosialylated Fcs. Please notice that monosialylated control Fc fragments do not bind sDC-SIGN, as they are largely devoid of di-sialylated glycans, consistent with above blotting experiments. Wash = no protein added, control for background binding. Fc and 4E10 binding was detected with horseradish peroxidase (HRP)-conjugated anti-Fc antibodies, while Fab binding was detected with HRP-conjugated anti-Fab antibodies.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.