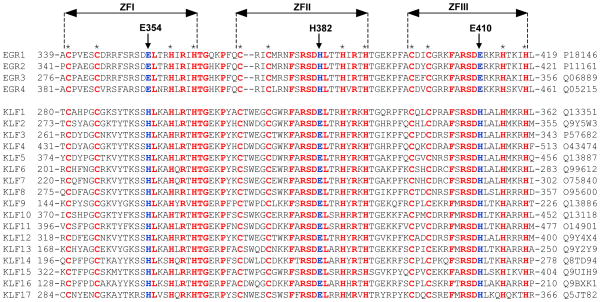
Figure 9.
Amino acid sequence alignment of DB domains of all known members of the human EGR1 family (EGR1-EGR4) and the related human KLF family (KLF1-KLF17). Absolutely conserved residues are shown in red, non-conserved residues at structurally-equivalent positions occupied by E354, H382 and E410 in EGR1 are colored blue, while all other residues are depicted in black. Each member is denoted by its acronym in the left column with the corresponding UniProt code provided in the right column for access to complete proteomic details on each member. The numerals denote the amino acid boundaries of DB domains for each member. The cysteine and histidine residues within each of the three C2H2-type zinc fingers of DB domains, denoted ZFI, ZFII and ZFIII, that coordinate the Zn2+ divalent ion in a tetrahedral arrangement are marked by asterisks. The vertical arrows indicate the position of E354/H382/E410 in EGR1 and their structural-equivalents in other DB domains.