Abstract
Pyriproxyfen is an insecticidal juvenile hormone analog that perturbs insect and tick development. Pyriproxyfen also alters parthenogenic reproduction in non-target cladoceran species as it induces male production that can lead to a decrease in fecundity, a reduction in population density, and subsequent ecological effects. In this study, we investigate the impacts of pyriproxyfen on Daphnia magna reproduction using a series of male production screening assays. These assays demonstrate that pyriproxyfen increases male production in a concentration-dependent fashion with an EC50 of 156 pM (50.24 ng L-1); a concentration considered environmentally relevant. Furthermore, pyriproxyfen decreases overall fecundity at all ages tested (7, 14, 21-d old female parthenogenic daphnids). Juvenile (3-d old) and reproductively mature (10-d old) female daphnids were also exposed to 155 pM pyriproxyfen for 2 – 12 d and reproduction measured for 16 d to compare the effects of short-term and prolonged exposures, and determine the potential for recovery. Results indicate that longer pyriproxyfen exposures (8–12 d) extend male production and decrease reproduction; however, daphnids exposed for only 2–4 d recover and produce a relatively normal abundance of neonates. In addition, juvenile daphnids are also very sensitive to pyriproxyfen, but the primary effect on juvenile daphnids is reduced reproduction and protracted development not male production. Taken together, continued use of pyriproxyfen around water bodies needs due caution because of its potential adverse effects with significant developmental delays and male production compounded by prolonged exposure.
Keywords: recovery, endocrine disruptor, environmental sex determination, cladocera, chronic toxicity
1. Introduction
Pyriproxyfen, (2-[1-methyl-2-(4-phenoxyphenoxy) ethoxy] pyridine,) is used in household, agricultural, and horticultural applications to control many insect species, including the common housefly (Musca domestica), mosquitos, imported red fire ants (Solenopsis invicta), and silverleaf whitefly (Bemisia argentifolii) (Sullivan and Goh, 2008). In addition, several over the counter topical applications for the control of flea and tick infestation in dogs and cats contain pyriproxyfen (Michael, 2005). Pyriproxyfen protects cats from recurrence of flea infestation for about six months (Maynard et al., 2001) although it does not kill adult fleas and ticks. This is because pyriproxyfen is a potent juvenile hormone agonist and endocrine disruptor (Sullivan and Goh, 2008) that inhibits larval development and maturation (Meola et al., 2000) as a juvenile hormone III mimic (Wang et al., 2005). Therefore, pyriproxyfen preferentially perturbs juvenile development and hatching success of flea and tick eggs.
Pyriproxyfen is relatively stable in the environment (Katagi and Takahashi, 1994), and is active in artificial Aedes aegypti breeding sites for 5 months (Darriet et al., 2010). Pyriproxyfen is also relatively lipophilic as it has an octanol/water partitioning coefficient of Kow 5.6 and a bioconcentration factor (BCF) of approximately 1500 in fish (Steginsky et al., 1994). Some models predict that pyriproxyfen may have a greater BCF in aquatic organisms with lower metabolic capacity based on its Kow (Meylan et al., 1999; Sullivan and Goh, 2008). The maximum aquatic pyriproxyfen concentrations are estimated to be about 290 pM (93ng L-1) based on a New York State risk assessment (Serafini, 2001), but may approach 1245 pM (400 ng L-1) 24 hours post-spray based on field tests (Schaefer and Miura, 1990). Consequently, there is concern that run-off from commercial and household applications could bioaccumulate and affect non-target species such as crustaceans (Tuberty and McKenney, 2005), especially parthenogenically reproducing cladocerans such as Daphnia (Olmstead and LeBlanc, 2003).
Daphnia are non-target, branchiopod crustaceans found in freshwater ponds all over the world. They are mid-level consumers and filter feeders that primarily eat algae and are preyed upon by larger arthropods and fish (Carpenter et al., 1987). There is a large body of research indicating their role in aquatic ecology (Ebert, 2005). Daphnia magna is commonly used in the assessment of environmental toxicants (OECD, 2012; Dang et al., 2012), which is due to its amenability to laboratory culture and parthenogenic reproduction. Under ideal conditions, Daphnia magna females can make clones of themselves with broods up to 50 neonates every 2–3 d (OECD, 2012).
However, under poor conditions such as overcrowding (Smith et al., 2009), poor food quality (Koch et al., 2009), shortage of food (Kleiven et al., 1992) or reduced photoperiod (Deng and Lynch, 1996), females produce males required for sexual reproduction. Sexual reproduction leads to the production and release of ephippia containing resting eggs that can survive difficult conditions including desiccation (Ebert, 2005). Most juvenile hormone analogs, including the endogenous crustacean juvenoid, methyl farnesoate, induce male production in Daphnia magna (Olmstead and LeBlanc, 2003; Tatarazako et al., 2003) and other cladoceran species (Oda et al., 2005b).
Pyriproxyfen is one of the most efficacious of these juvenile hormone analogs, and consequently it is included in the USEPA draft list for Tier 1 screening of endocrine disrupting chemicals (USEPA, 2007). In Daphnia magna, pyriproxyfen's male production EC50 is about 170 – 310 pM (55 – 100ng L-1) (Olmstead and LeBlanc, 2003; Matsumoto et al., 2008). Therefore, environmentally relevant concentrations could increase male production in Daphnia and other cladocerans. Increases in male production in Daphnia species decreases the output of parthenogenic females, reduces population growth rate, and ultimately population abundance (Olmstead and LeBlanc, 2003). Daphnids play a crucial role as keystone species in aquatic ecosystems as primary consumers of algae and a source of food for larger invertebrates and fish (Gaedke and Straile, 1998). Perturbations in Daphnia abundance could lead to environmental problems such as algal blooms, reduced fish populations, and overall disruption of the aquatic ecosystem. Therefore, pyriproxyfen must be sprayed with caution around aquatic environments (Serafini, 2001; Sullivan and Goh, 2008).
The ability of an organism to recover is crucial to ecosystem health, and it has been proposed that early stage exposure to endocrine disruptors may permanently damage endocrine systems and contribute to reproductive and carcinogenic effects in adults (Nichols et al., 2011; Chen et al., 2012). If individuals are unable to recover from toxic insults than recovery of ecosystems may be perturbed. It has been suggested that male production assays could be used as tier 2 screens for the USEPA's Endocrine Disruptor Screening Program (Wang et al., 2005), and this screen and other screens if modified could be used to assess the ability of organisms to recover from exposure to endocrine disruptors. Therefore, the reproductive and male producing effects of acute and chronic exposures to pyriproxyfen were examined in juvenile and adult D. magna. We investigated the reproductive toxicity and male producing effects of pyriproxyfen to Daphnia magna, and tested whether acute (2–4 d) and chronic (8–12 d) exposures have similar effects on juvenile and reproductively mature daphnids. In addition, we determined whether daphnids recover from pyriproxyfen exposures, and if recovery was dependent on age and length of exposure. Overall, the purpose of this manuscript is to compare the reproductive effects of pyriproxyfen on Daphnia magna, determine if Daphnia can recover from exposure to this juvenile hormone analog, and better define the potential environmental impacts of acute and chronic exposures of pyriproxyfen to Daphnia magna by investigating a couple of different developmental stages.
2. Materials & Methods
2.1. Daphnia magna culture
A strain of Daphnia magna has been maintained within the Environmental Toxicology program at Clemson University for about 20 years, and cultured as described previously (Baldwin et al., 2001). Daphnia were cultured in standard moderately hard water with a pH of 8.2–8.4 and a 16:8 light: dark cycle at 20–22 °C. Adult Daphnia were fed 6 × 106 Pseudokirchneriella subcapitata per daphnid/d supplemented with 0.25 mg dry weight of blended TETRAFIN fish flakes (catalog # 46798-16140; Tetra Holding Inc., VA) in a 50 L aqueous suspension.
2.2.1. Male Production Assays: Pyriproxyfen Concentration-Response
Fourteen d-old female Daphnia magna (n = 10) were placed in individual 50 ml glass beakers containing 40 ml of moderately hard water. Daphnids were exposed to pyriproxyfen (99% purity; FLUKA, analytical, Chemie, Buchs, Switzerland,) dissolved in absolute ethanol provided to Daphnia cultures at 0.02% of the media. The untreated group received only absolute ethanol at 0.02%. Culture media was changed every other d.
The sex of each neonate following pyriproxyfen exposure at 0 – 1245 pM (0 – 400 ng L-1) was assessed after each brood based on the length of the first antennae (Olmstead and LeBlanc, 2003) using a dissecting microscope (American Optical-150W haloid cold light source). Male production and overall fecundity was assessed for the first four broods, which takes approximately 12 d. However, the first brood was eliminated from the data because the presence of males in this brood is sporadic and the first brood is often exposed to pyriproxyfen after the specific developmental timeframe necessary to alter the sex of the developing egg (Olmstead and LeBlanc, 2002; Kato et al., 2011).
2.2.2. Male Production Assays: Effects of Age on Male Production
Adult female daphnids (n = 10) of different ages (7, 14, 21-d old) were exposed to pyriproxyfen at 155 pM (50 ng L-1). This is a low but effective concentration of pyriproxyfen, near the EC50 for inducing male production, and within the estimated environmental concentrations of 90 – 290 pM at aquatic depths of 1 – 6 feet (Serafini, 2001), and lower than measured concentrations (up to 1245 pM) 24 hours after treatment of a rice field plot (Schaefer and Miura, 1990). Seven-d old adult females just prior to producing their initial brood, 14-d old adult females near their reproductive peak, and 21-d old adult females that are typically showing reduced fecundity because of their advanced age were individually exposed to pyriproxyfen. The sex of each neonate following pyriproxyfen exposure was assessed based on the length of the first antennae (Olmstead and LeBlanc, 2003) in broods 2–4 as described above. The ratio of males to females were examined instead of absolute numbers as the 7-d old and 21-d old daphnids typically produce less offspring than the 14-d old female daphnids, which makes direct comparisons problematic.
2.2.3. Acute and Chronic Pyriproxyfen Exposure: Comparison of Juvenile and Adult Exposures
Parthenogenic female daphnids age 3-d (juvenile) or 10-d old (reproductively mature) were exposed to 155 pM (50 ng L-1) pyriproxyfen for 0, 2, 4, 8, or 12 d. The overall fecundity and male production of the unexposed and exposed daphnids was followed for 18 d after the initial exposures in the 3-d old daphnids, and for 16 d following the initial exposures in the 10-d old daphnids. Initial reproduction (or delayed onset of reproduction), initial production of males, and the cessation of male production was also noted. The purpose of this experiment was to assess the persistent effects of pyriproxyfen on fecundity and male production, and to determine the ability of daphnids to recover from pyriproxyfen exposures of different time lengths.
2.3. Statistics
Statistical differences were determined by ANOVA followed by Dunnett's test using GraphPad Prism Version 4.3 (GraphPad Software La Jolla CA, USA), except when multiple different groups were compared and then statistical differences were determined by ANOVA followed by Tukey's multiple comparison tests using GraphPad Prism. EC50 values were determined from log transformed data using GraphPad statistical software as described previously (Baldwin and Roling, 2009).
3. RESULTS
3.1. Pyriproxyfen Concentration-Response
Pyriproxyfen is known to induce male production in cladoceran species (Oda et al., 2005b) with an EC50 of approximately 170 – 310 pM in D. magna (Olmstead and LeBlanc, 2003; Matsumoto et al., 2008). However, pyriproxyfen's concentration-response curve is steep and effective concentrations may vary by laboratory and strain (Wang et al., 2005). Pyriproxyfen decreased overall fecundity (the production of males and females) and increased male production in a concentration-dependent manner (Fig. 1). The EC50 value for male production is 158 pM (95% CI of 134 – 182 pM) similar to previous studies with D. magna (Olmstead and LeBlanc, 2003; Matsumoto et al., 2008). The Lowest Observed Effect Concentration (LOEC) for fecundity is 78 pM (25 ng L-1) (Fig. 1), and the LOEC for male production is 155 pM (50 ng L-1). Pyriproxyfen is estimated to reach aquatic concentrations of about 300 pM following run-off after application (Serafini, 2001; Olmstead and LeBlanc, 2003). Concentrations nearly half the relevant environmental concentrations (155 pM) consistently and significantly perturbed fecundity and increased male production, and consequently we used 155 pM in our subsequent studies on the time- and age-dependent effects of pyriproxyfen.
Fig. 1. Influence of pyriproxyfen on overall fecundity and male production.
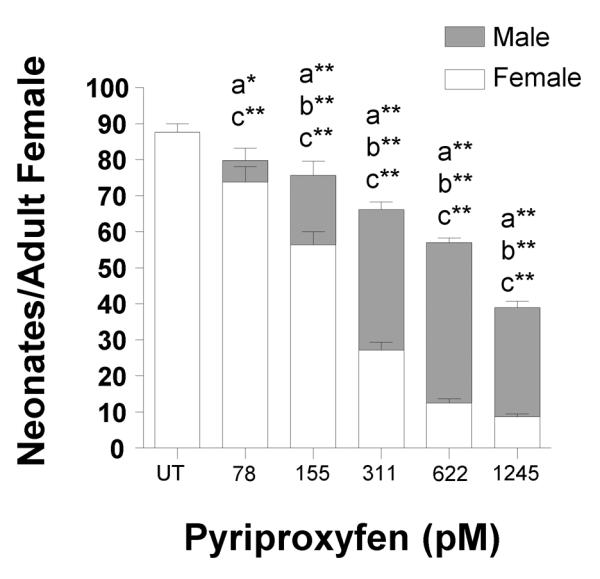
The number of male and female neonates produced per adult female. Data are shown as mean ± SEM. (a) Indicates a significant difference in the total number of neonates produced, (b) indicates a significant difference in the number of male neonates produced, and (c) in dicates a significant difference in the total number of female neonates produced compared to the untreated (UT) control. Statistical differences were analyzed by ANOVA followed by Dunnett's multiple comparison test and an (*) indicates p < 0.05 and (**) indicates p < 0.01 (n = 10).
3.2. Effects of Age on Male Production
Exposure of 7, 14, and 21-d old female D. magna to 155 pM pyriproxyfen induces male production in adult daphnids regardless of age and at similar proportions (Fig. 2) although 14-d old daphnids typically produce the greatest number of offspring overall (data not shown). Brood-wise comparisons (examining broods 2–4 individually) indicate that older animals (21-d old) respond to pyriproxyfen quicker (Suppl File 1); producing a greater percentage and number of males in the second brood than the younger daphnids in both assays.
Fig. 2. Effects of age on the sensitivity of D. magna to pyriproxyfen.
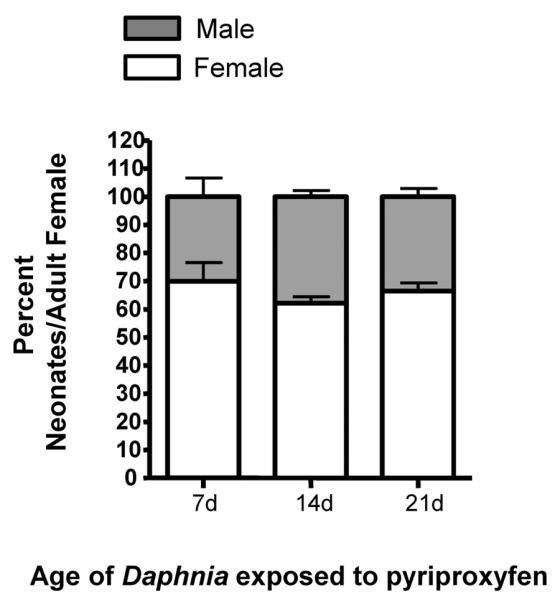
The percent of male and female neonates per adult female were quantified at different ages. Data are shown as mean ± SEM. Statistical differences were analyzed by ANOVA followed by Dunnett's multiple comparison test (n = 10).
3.3. Acute and Chronic Pyriproxyfen Exposure: Comparison of Juvenile and Adult Exposures
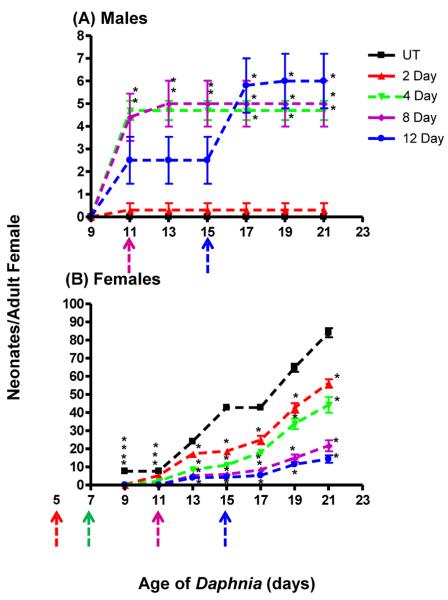
Exposure of female juvenile Daphnia (3-d old) to 155 pM pyriproxyfen for 0, 2, 4, 8, or 12-d perturbed male production, delayed the onset of reproduction, and reduced overall fecundity in a time-dependent manner. Pyriproxyfen increased the production of males after four or more d of exposure (Fig. 3A). The 2-d exposures produced very few males (only one female produced 3 males) and the 2-d exposure group data are significantly different from the groups exposed for more than 2 ds (p < 0.001). Overall, the effects of pyriproxyfen on male production in juveniles are not efficacious. Pyriproxyfen-treated females produced no more than six males per female, and male production was primarily relegated to the early broods even when exposure occurred well into adulthood with the exception of the daphnids exposed for 12 d (Fig. 3A).
Fig. 3. Temporal effects of periodic exposure to pyriproxyfen on reproduction in three-d old Daphnia magna:
Female Daphnia magna (3-d old) were exposed to 155 pM pyriproxyfen for 2, 4, 8 or 12 d and reproduction quantified to determine the time-dependent effects of pyriproxyfen on the number of male (A) and female neonates (B) produced. Data are shown as mean ± SEM. Statistical differences were analyzed by ANOVA followed by Tukey's multiple comparison test and an (*) indicates p < 0.001 compared to the untreated (UT) Daphnia (n = 10). Colored arrows indicate the age in which pyriproxyfen exposure is withdrawn (8 and 12 d exposures).
The most potent effect of pyriproxyfen exposure to juveniles is on female production and overall fecundity. Pyriproxyfen reduced initial brood size and delayed reproduction (Fig. 3B; p < 0.001 ANOVA for 9-d old daphnids) relative to the unexposed daphnids, indicating that any exposure (acute or chronic) to pyriproxyfen slows maturity and reduces initial fecundity. D. magna usually deposit eggs in the brood chamber at approximately 6–7 d of age. The eggs develop into neonates over a 2–3 d period and are released at first molt, which occurs at about d nine (Olmstead and LeBlanc, 2002; Kato et al., 2011). We observed that the pyriproxyfen-exposed daphnids showed a delay in the release of eggs to the brood chamber, and in turn the pyriproxyfen-exposed daphnids did not reproduce until d 11 while unexposed daphnids reproduced by d nine (Fig. 3). Consequently, broods 1–3 showed the most pronounced effects from pyriproxyfen exposure (Fig. 3; Suppl File 2).
There is also a significant exposure time-dependent decrease in the production of females and overall fecundity following pyriproxyfen exposure (Fig. 3B). The chronic exposures (more than 4 d) caused significantly lower fecundity compared to the acute exposures. Each increase in exposure length caused a statistically greater reduction in fecundity than the corresponding treatments except when comparing the daphnids exposed for 8 d to the daphnids exposed for 12 d. These groups show nearly equal drops in fecundity (statistics not shown; ANOVA followed by Tukey's). Typically, the longer the exposure to pyriproxyfen the greater the decline in fecundity with an 80% decrease in fecundity in daphnids exposed 12 d compared to unexposed daphnids (Fig. 4).
Fig. 4. Overall effects of temporary exposures to pyriproxyfen on reproduction in three-d old Daphnia magna:
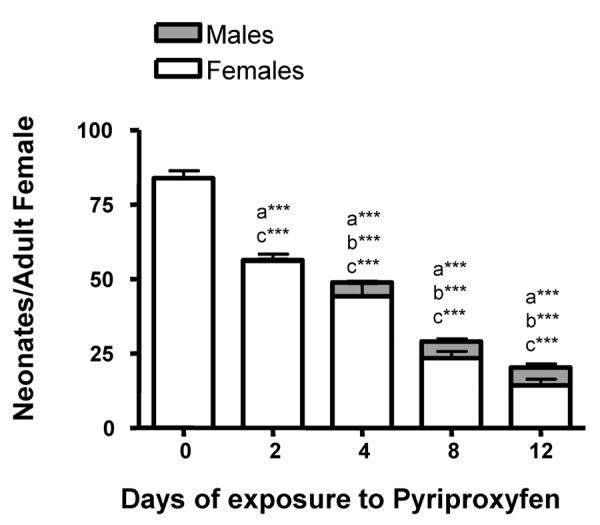
Female Daphnia magna that were 3-d old were exposed to 155 pM pyriproxyfen for 2, 4, 8, or 12 d and reproduction monitored for 18 d. Data on the number and sex of the neonates produced by pyriproxyfen-exposed Daphnia are shown as mean ± SEM. (a) Indicates a significant difference in the total number of neonates produced, (b) indicates a significant difference in the number of male neonates produced, and (c) indicates a significant difference in the total number of female neonates produced compared to untreated (UT) Daphnia magna. Statistical differences were analyzed by ANOVA followed by Dunnett's multiple comparison test and an (*) indicates p < 0.05, (**) indicates p < 0.01 (***) indicates p < 0.001 (n = 10).
Reproduction appeared to recover in the 2- and 4-d pyriproxyfen exposure groups by d 17 (approximately 10-d after pyriproxyfen withdrawal) with reproductive slopes similar to the untreated Daphnia (Fig. 3B). Reproduction within the first three broods was clearly reduced at all exposure lengths and in a time-dependent manner. However, the daphnids exposed for only 2–4 d fully recovered during the last three broods (Suppl File 2). Daphnids exposed for 8 or 12-d did not recover. Daphnids exposed for 8-d showed lower fecundity and daphnids exposed for 12-d showed lower fecundity coupled with continued male production (Suppl File 2B).
Overall, the primary effect of pyriproxyfen on juvenile daphnids is lower fecundity as pyriproxyfen reduced the total number of neonates and the number of females produced by 3-d old Daphnia in a time-dependent manner (Fig. 4). Some of pyriproxyfen's effects on fecundity can be explained by delayed development (Fig. 3). The effect of juvenile hormones on daphnid reproduction and juvenile development has not been as well documented to our knowledge.
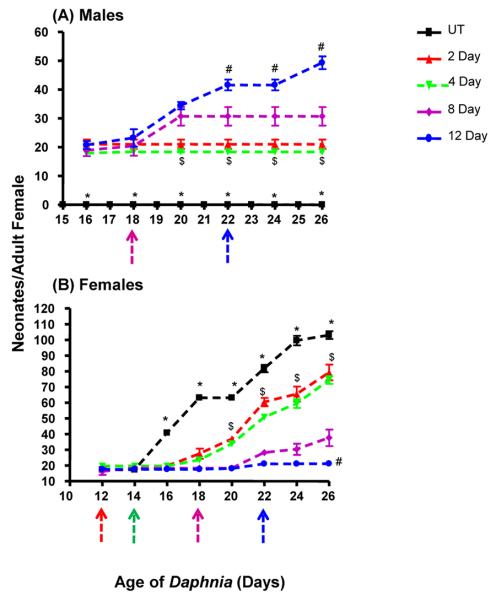
Similarly, we determined the time-dependent influence of 0, 2, 4, 8, and 12-d exposures of 155 pM pyriproxyfen on 10-d old Daphnia. Male production occurred six-d after the initial exposure in all the treatment groups (Fig. 5A). Initial male production was equal in all groups; however, over the course of the assay, male production increased in a time-dependent fashion (Fig. 5A). The Daphnia exposed for 2–4 d demonstrated relatively quick recovery compared to the 8 or 12 d exposure groups as 2–4 d exposed daphnids quit producing males about 2–4 d after the pyriproxyfen exposure ceased (Fig. 5A). This finding indicates that the male-producing effects of pyriproxyfen are reversible, and longer exposures significantly increase the likelihood of an adverse outcome.
Fig. 5. Temporal effects of periodic exposure to pyriproxyfen on reproduction in ten-d old Daphnia magna:
Female Daphnia magna (10-d old) were exposed to 155 pM pyriproxyfen for 2, 4, 8 or 12 d and reproduction quantified to determine the time-dependent effects of pyriproxyfen on the number of male (A) and female neonates (B) produced. Data are shown as mean ± SEM. Statistical differences from the untreated daphnids were determined by ANOVA followed by Tukey's multiple comparison test. An (*) indicates all treated groups are different from the untreated (UT) group (p < 0.001), ($) indicates a significant difference between the groups treated for 2–4 d and the other groups, and (#) indicates a significant difference between the group treated for 12-d and all the other groups (p < 0.001) (n = 10). Colored arrows indicate the age in which pyriproxyfen exposure is withdrawn.
While male production increased, female production decreased (Fig. 5B) providing fewer Daphnia for future parthenogenic reproduction. Female production decreased in a time-dependent manner (Fig. 5B); however, total fecundity was only perturbed following the chronic (8–12 d) exposures (Fig. 5, 6). The data suggest that the primary effect on female production was early but not immediate; about 6–8 d after exposure. Recovery was observed in the 2 – 4 d exposure groups shortly thereafter (Fig. 5B).
Therefore, we separated the data and examined male production, female production, and overall fecundity during the first three and last three broods. Male production does not differ between treatment groups (untreated group excluded) during the initial broods, but only the daphnids exposed for 12-d showed male production in the last three broods (Suppl File 3). Daphnia exposed to pyriproxyfen for 8-d recovered enough to quit producing males, but exposures that lasted 12 d significantly perturbed fecundity and this effect was particularly evident in the last three broods (Fig. 5B; Suppl File 3). This finding indicates that adult daphnids can recover from acute exposures to pyriproxyfen, whereas continued exposure causes irreversible loss of fecundity.
Overall, the primary effects of pyriproxyfen on mature 10-d old daphnids are increased male production and lower fecundity (Fig. 5, 6). The magnitude of the effects of pyriproxyfen on adults is time-dependent, and the total number of offspring is only perturbed in reproductively active female daphnids if exposure lasted longer than 4-d (Fig. 6). However, the sex ratios of the offspring are perturbed. The percent of male offspring exposed to pyriproxyfen for 2, 4, 8, or 12-d was 20%, 20%, 45%, and 50%, respectively (Fig. 6). Adult female daphnids fully recovered less than 4-d after pyriproxyfen exposure ceased with the exception of the daphnids exposed 12-d. In contrast, juvenile daphnids exposed for only 2 – 4 d needed approximately 10 d for recovery. In summary, male production is much greater in the exposed adults, but overall fecundity is perturbed more in the exposed juveniles probably due in part to delayed maturity, indicating that pyriproxyfen has different effects on juvenile daphnids than mature daphnids.
Fig. 6. Overall effects of temporary exposures to pyriproxyfen on reproduction in ten-d old Daphnia magna:
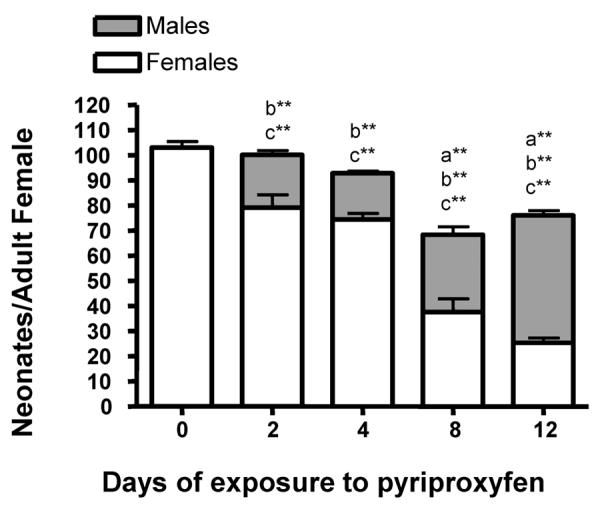
Female Daphnia magna that were ten-d old were exposed to 155 pM pyriproxyfen for 2, 4, 8, or 12 d and reproduction monitored for 16 d. Data on the number and sex of the neonates produced are shown as mean ± SEM. (a) Indicates a significant difference in the total number of neonates produced, (b) indicates a significant difference in the number of male neonates produced, and (c) indicates a significant difference in the total number of female neonates produced compared to untreated (UT) Daphnia magna. Statistical difference were analyzed by ANOVA followed by Dunnett's multiple comparison test and an (*) indicates p < 0.05 and (**) indicates p < 0.01 (n = 10).
4. DISCUSSION
Acute exposures to pyriproxyfen reduced fecundity, increased time to maturity, and increased male production. This could have profound effects on the population of Daphnia in an aquatic ecosystem. The effect of pyriproxyfen or juvenile hormone analogs on population recovery has not been investigated; however, the effects of other pesticides such as cypermethrin, fenvalerate, and paraoxon-methyl on Daphnia population recovery have been investigated. Some studies have indicated that reduced brood size and increased time to reproductive maturity may not perturb intrinsic growth rate of the population (Kim et al., 2008). However, most studies indicate that perturbations in reproduction may take 1–3 generations for recovery (Liess et al., 2006; Pieters and Liess, 2006) and even longer (5 or more generations) if there are other stressors such as a population in stasis, poor nutrition, competition, or delayed life history events (Liess et al., 2006; Foit et al., 2012). Competition for resources by non-reproducing males and delayed reproduction are both concerns with pyriproxyfen. These variables can also continue to perturb population structure after population numbers have recovered (Liess et al., 2006).
Interestingly, the adverse effects of pyriproxyfen exposure to juvenile and reproductively mature daphnids are different. Juvenile daphnids primarily show reduced fecundity and delayed maturity; adult daphnids show limited reduced fecundity and much greater male production. Two-d exposures to juvenile daphnids did not induce male production with the exception of one individual and this daphnid only produced three males. We hypothesize that pyriproxyfen may not induce male production in juvenile daphnids because the ovaries are not mature enough to produce offspring. The receptors or requisite transcription factors necessary to produce males may not be expressed until near reproductive maturity. Interestingly, the male producing effects of juvenoids including pyriproxyfen on reproductively mature Daphnia are probably unique to Cladocera (Olmstead and LeBlanc, 2003; Oda et al., 2005a). However, the effects of pyriproxyfen on juveniles are similar to those observed in insects and ticks in which reproductive maturity or metamorphosis is inhibited, and the individuals are kept in the juvenile stages (Ishaaya and Horowitz, 1992; Fathpour et al., 2007).
The effects of 155 pM pyriproxyfen on adults are significant, but reproduction was perturbed more in the exposed juvenile Daphnia compared to the adult Daphnia, especially following the shorter exposures. Exposed juveniles took longer to recover and their initial reproduction was significantly delayed (Fig. 3). Mature daphnids showed no loss of fecundity in the first three broods; just a significant increase in male production (Suppl File 3). Furthermore, only the mature daphnids exposed for a longer period of time demonstrated reduced fecundity (Fig. 6). However, that does not take into account the multigenerational effects male production has on population numbers as only the females can reproduce parthenogenically and accordingly produce high numbers of offspring. Therefore, Daphnia numbers are likely to drop significantly even in the adult, acute exposure groups because of reduced reproductive capacity of future generations.
Other crustaceans (and sensitive beneficial insects) may also be adversely affected as pyriproxyfen has been shown to decrease survival rates in decapod species such as shrimp (Tuberty and McKenney, 2005), and induce abnormal ovarian function in Island Red Crab (Linton et al., 2009). Pyriproxyfen applied to rice plots at 0.05 and 0.11 Kg active ingredient/hectare was persistent in the water column at 0.5 m depths for 2 d. Residues were detected at 400 ng L-1 (1245 pM) after 24 hours and in turn caused a decline in the populations of Podocopa (subclass of ostracods), Odonata (damselflies and dragonflies), and Cladocera (Schaefer and Miura, 1990). Furthermore, Odonata and Chironomidae died during pupal-adult ecdysis in subsequent tests (Schaefer and Miura, 1990). Pyriproxyfen also produced minor morphogenic aberrations in Odonata and reproductive suppression in Cladocera and Ostracoda (Schaefer et al., 1988).
Given that pyriproxyfen has an average half-life of 5.04 d in water (Sullivan, 2000), and 16 to 21 d in sediments (WHO, 2008), the effects on Cladocera or other non-target crustaceans may be mitigated due to recovery. Multiple seasonal sprayings can be helpful in controlling pests with multiple generations per season and are sometimes suggested. However, follow-up spraying may have severe effects on aquatic communities because reproduction is perturbed in a time-dependent manner, and juvenile daphnids, which already show delayed development following acute exposures, may not recover from chronic exposures (Fig. 4, 6). Recent data indicates that follow-up spraying is not necessary as pyriproxyfen is effective for 5 months and even longer if used in combination with spinosad (Darriet et al., 2010);(Chen et al., 2008).
We also investigated the age-dependent effects of pyriproxyfen on male production at 155 pM, examining adolescent (7-d old), reproductively mature (14-d old), and reproductively declining daphnids (21-d old). These assays reveal that all adolescent and adult stages are sensitive to the male producing effects of pyriproxyfen. Overall, the data indicate that age has little effect on the ratio of male/female production. An interesting and repeatable effect observed is increased production of males in brood 2 in the oldest (21-d old) daphnids compared to the younger daphnids (Suppl File 1). Previous research indicates that D. pulex offspring become more male-biased with maternal age during overcrowding (Fitzsimmons and Innes, 2006). We did not observe an increase in the male production or the male/female ratio over several broods, but did observe an increased initial sensitivity to a stressor that induces male production. Increased male production in older daphnids may provide a mechanism by which a population of Daphnia can respond to a key stressor by producing males, while some younger individuals may continue to produce primarily parthenogenic clones in order to increase or stabilize population numbers.
In conclusion, the male producing effects of juvenoids including pyriproxyfen on Daphnia are probably unique to Cladocera (Olmstead and LeBlanc, 2003; Oda et al., 2005b). However, the effects of pyriproxyfen on juveniles are similar to those observed in insects in which reproductive maturity is inhibited (Ishaaya and Horowitz, 1992; Fathpour et al., 2007). Furthermore, pyriproxyfen suppressed fecundity more and recovery took longer in the juvenile daphnids compared to the mature daphnids. Daphnids, especially adults, can recover from the reproductive toxicity elicited by pyriproxyfen after short-term exposures. However, the effects of pyriproxyfen on non-target Daphnia species (Schaefer et al., 1988) are substantial and may include other arthropods at environmentally relevant concentrations (Schaefer and Miura, 1990). Consequently, our data indicate that while pyriproxyfen is a relatively safe pesticide from which individuals can recover; its effects can occur at concentrations within the expected environmental concentrations. Further, the effects are time-dependent and individuals may not recover from chronic exposures.
Supplementary Material
Highlights.
Pyriproxyfen induces male production in adult D. magna with an EC50 of 156 pM
Pyriproxyfen primarily reduces reproduction and slows development in juveniles
Pyriproxyfen's effects on fecundity are more pronounced in juveniles than adults
Recovery takes longer in juveniles than adults
The length of exposure is crucial as daphnids can recover from acute exposures
Acknowledgements
The authors would like to thank Dr. Patrick Gerard for his statistical advice. Funds for this project were provided by Clemson University start-up, a George R. MacDonald Fellowship to Gautam Ginjupalli, and National Institutes of Environmental Health Sciences grant R15-ES017321 to William Baldwin.
Abbreviations
- (pM)
picomolar
- (EC50)
half-maximal effect concentration
- (ng L-1)
nano grams/Liter
- (USEPA)
United States Environmental Protection Agency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin WS, Bailey R, Long KE, Klaine S. Incomplete ecdysis is an indicator of ecdysteroid exposure in Daphnia magna. Environ Toxicol Chem. 2001;20:1564–1569. [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol Sci. 2009;107:93–105. doi: 10.1093/toxsci/kfn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, Kitchell J, Hodgson J, Cochran P, Elser J, Elser M, Lodge D, Kretchmer D, He X, Von E. Regulation of lake primary productivity by food web structure. Ecology. 1987;68:1863–1876. doi: 10.2307/1939878. [DOI] [PubMed] [Google Scholar]
- Chen C, Andy-Tana W, Loke S, Lee H, Yasmin A, Sofian-Azirun M. Effectiveness of pyriproxyfen-controlled release block against larvae of Aedes (Stegomyia) aegypti in Kuala Lumpur, Malaysia. Dengue Bulletin. 2008;32:199–206. [Google Scholar]
- Chen W, Fu X, Dong B, Wang Y, Shiah S, Moore D, Huang W. Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology. 2012;56:1499–1509. doi: 10.1002/hep.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriet F, Marcombe S, Etienne M, Yébakima A, Agnew P, Yp-Tcha M, Corbel V. Field evaluation of pyriproxyfen and spinosad mixture for the control of insecticide resistant Aedes aegypti in Martinique (French West Indies) Parasites & Vectors. 2010;3 doi: 10.1186/1756-3305-3-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z, Cheng Y, Chen HM, Cui Y, Yin HH, Traas T, Montforts M, Vermeire T. Evaluation of the Daphnia magna reproduction test for detecting endocrine disruptors. Chemosphere. 2012;88:514–23. doi: 10.1016/j.chemosphere.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Deng H, Lynch M. Change of genetic architecture in response to sex. Genetics. 1996;143:203–212. doi: 10.1093/genetics/143.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. National Center for Biotechnology Information; Bethesda, MD: 2005. [Google Scholar]
- Fathpour H, Noori A, Zeinali B. Effects of a juvenoid pyriproxyfen on reproductive organ development and reproduction in German Cockroach (Dictyoptera:Blattellidae). Iranian J Sci & Technol Trans-A. 2007;31:89–98. [Google Scholar]
- Fitzsimmons JM, Innes DJ. Inter-genotype variation in reproductive response to crowding among Daphnia pulex. Hydrobiologia. 2006;568:187–205. [Google Scholar]
- Foit K, Kaske O, Leiss M. Competition increases toxicant sensitivity and delays the recovery of two interacting populations. Aquat Toxicol. 2012;106–107:25–31. doi: 10.1016/j.aquatox.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Gaedke U, Straile D. Daphnids: Keystone species for the pelagic food web structure and energy flow. - A body size-related analysis linking seasonal changes at the population and ecosystem levels. Adv Limnol. 1998;53:587–610. [Google Scholar]
- Ishaaya I, Horowitz A. Novel phenoxy juvenile hormone analog (pyriproxyfen) suppresses embryogenesis and adult emergence of sweetpotato whitefly (Homoptera: Aleyrodidae. J Econ Entomol. 1992;85:2113–2117. [Google Scholar]
- Katagi T, Takahashi N. Hydrolysis of S-31183 in Buffered Aqueous Solutions. Sumitomo Chemical Company; 1994. [Google Scholar]
- Kato Y, Kobayashi K, Watanabe H, Iguchi T. Environmental sex determination in the branchiopod crustacean Daphnia magna: Deep conservation of a doublesex gene in the sex-determining pathway. PLoS Genetics. 2011;7:e1001345. doi: 10.1371/journal.pgen.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Jung J, Oh S, Choi K. Aquatic toxicity of cartap and cypermethrin to different life stages of Daphnia magna and Oryzias latipes. J Environ Sci & Health Part-B. 2008:56–64. doi: 10.1080/03601230701735029. [DOI] [PubMed] [Google Scholar]
- Kleiven OT, Larsson P, Hobæk A. Sexual Reproduction in Daphnia magna requires three stimuli. OIKOS. 1992;65:197–206. [Google Scholar]
- Koch U, Von Elert E, Straile D. Food quality triggers the reproductive mode in the cyclical parthenogen Daphnia (Cladocera) Oecologia. 2009;159:317–324. doi: 10.1007/s00442-008-1216-6. [DOI] [PubMed] [Google Scholar]
- Liess M, Pieters B, Duquesne S. Long-term signal of population disturbance after pulse exposure to an insecticide: rapid recovery of abundance, persistent alteration of structure. Environ Toxicol Chem. 2006;25:326–1331. doi: 10.1897/05-466r.1. [DOI] [PubMed] [Google Scholar]
- Linton S, Lauren D, Harman L. Potential endocrine disruption of ovary synthesis in the Christmas Island red crab Gecarcoidea natalis by the insecticide pyriproxyfen. Comp Biochem Physiol A Mol Integr Physiol. 2009;154:289–297. doi: 10.1016/j.cbpa.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ikuno E, Itoi S, Sugita H. Chemical sensitivity of the male daphnid, Daphnia magna, induced by exposure to juvenile hormone and its analogs. Chemosphere. 2008;72:451–456. doi: 10.1016/j.chemosphere.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Maynard L, Houffschmitt P, Lebreux B. Field efficacy of a 10 per cent pyriproxyfen spot-on for the prevention of flea infestations on cats. J Small Anim Pract. 2001;42:491–494. doi: 10.1111/j.1748-5827.2001.tb02454.x. [DOI] [PubMed] [Google Scholar]
- Meola R, Meier K, Dean S, Bhaskaran G. Effect of Pyriproxyfen in the Blood Diet of Cat Fleas on Adult Survival, Egg Viability, and Larval Development. J Med Entomol. 2000;37:503–506. doi: 10.1603/0022-2585-37.4.503. [DOI] [PubMed] [Google Scholar]
- Meylan WM, Howard PH, Boethling RS, Aronson D, Printup H, Gouchie S. Improved method for estimating bioconcentration/bioaccumulation factor from octanol/water partition coefficient. Environ Toxicol Chem. 1999;18:664–672. [Google Scholar]
- Michael KR. Advances in the control of Ctenocephalides felis (cat flea) on cats and dogs. Trends in Parasitol. 2005;21:232–236. doi: 10.1016/j.pt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Nichols J, Breen M, Denver R, Distefano J, Edwards J, Hoke R, Volz D, Zhang X. Predicting chemical impacts on vertebrate endocrine systems. Environ Toxicol Chem. 2011;30:39–51. doi: 10.1002/etc.376. [DOI] [PubMed] [Google Scholar]
- Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in Daphnia magna (Cladocera, Crustacea) exposed to juvenile hormones and their analogs. Chemosphere. 2005a;61:1168–1174. doi: 10.1016/j.chemosphere.2005.02.075. [DOI] [PubMed] [Google Scholar]
- Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in four cladoceran species exposed to a juvenile hormone analog, fenoxycarb. Chemosphere. 2005b;60:74–78. doi: 10.1016/j.chemosphere.2004.12.080. [DOI] [PubMed] [Google Scholar]
- OECD . Daphnia magna Reproduction Test,OECD Guidelines for the Testing of Chemicals. OECD Publishing; 2012. pp. 1–25. [Google Scholar]
- Olmstead AW, LeBlanc GA. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool. 2002;293:736–739. doi: 10.1002/jez.10162. [DOI] [PubMed] [Google Scholar]
- Olmstead AW, LeBlanc GA. Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean Daphnia magna. Environ Health Perspect. 2003;111:919–924. doi: 10.1289/ehp.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters B, Liess M. Population developmental stage determines the recovery potential of Daphnia magna populations after fenvalerate application. Environ Sci Technol. 2006;40:6157–6162. doi: 10.1021/es052180o. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Miura T. Chemical persistence and effects of S-31183, 2- 1-methyl-2-(4-phenoxyphenoxy)ethoxy pyridine, on aquatic organisms in field-tests. J Econ Entomol. 1990;83:1768–1776. [Google Scholar]
- Schaefer C, Miura T, Dupras E, Mulligan F, Wilder W. Efficacy, nontarget effects and persistence of S-31183, a promising mosquito (Diptera-Culicidae) control agent. J Econ Entomol. 1988;86:1648–1655. doi: 10.1093/jee/81.6.1648. [DOI] [PubMed] [Google Scholar]
- Serafini M. In: Pyriproxyfen - Registration of a Major Change of Label for Esteem® 3/01. Division of Solid & Hazardous Materials, P.P.R.S., editor. New York State Department of Environmental Conservation; New York: 2001. [Google Scholar]
- Smith A, Acharya K, Jack J. Overcrowding, food and phosphorus limitation effects on ephippia production and population dynamics in the invasive species Daphnia lumholtzi. Hydrobiologia. 2009;618:47–56. [Google Scholar]
- Steginsky C, Powell J, Chang J. Characterization of 14C Residues in Bluegill Sunfish Treated With 14C Pyriproxyfen Data Package Report No.156629-N, DPR No.52080-0041(Unpublished) Sumitomo Chemical Company; 1994. [Google Scholar]
- Sullivan J. Environmental fate of Pyriproxyfen. California Department of Pesticide Regulation; 2000. [Google Scholar]
- Sullivan J, Goh K. Environmental fate and properties of pyriproxyfen. J Pest Sci. 2008;33:339–350. [Google Scholar]
- Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere. 2003;53:827–833. doi: 10.1016/S0045-6535(03)00761-6. [DOI] [PubMed] [Google Scholar]
- Tuberty SR, McKenney CL. Ecdysteroid responses of estuarine crustaceans exposed through complete larval development to juvenile hormone agonist insecticides. Integr Comp Biol. 2005;45:106–117. doi: 10.1093/icb/45.1.106. [DOI] [PubMed] [Google Scholar]
- USEPA . Draft List Of Initial Pesticide Active Ingredients And Pesticide Inerts To Be Considered For Screening UnderThe Federal Food, Drug, And Cosmetic Act U.S. Environmental Protection Agency. In: USEPA, editor. Draft List of Chemicals for Initial Tier 1 Screening. USEPA; 2007. p. 18. [Google Scholar]
- Wang HY, Olmstead AW, Li H, LeBlanc GA. The screening of chemicals for juvenoid-related endocrine activity using the water flea Daphnia magna. Aquat Toxicol. 2005;74:193–204. doi: 10.1016/j.aquatox.2005.05.010. [DOI] [PubMed] [Google Scholar]
- World Health Organization . In: Pyriproxyfen in Drinking-water:Use for Vector Control in Drinking-water Sources and Containers. WHO, editor. Geneva: 2008. pp. 1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.