Abstract
Glutamatergic pathways are a major information-carrying and -processing network of inputs in the brain. There is considerable evidence suggesting that glutamatergic pathways do not represent a homogeneous group and that they can be segregated into at least two broad categories. Class 1 glutamatergic inputs, which are suggested to be the main information carriers, are characterized by a number of unique synaptic and anatomical features, such as the large synaptic boutons with which they often terminate. On the other hand, Class 2 inputs, which are thought to play a modulatory role, are associated, amongst other features, with exclusively small terminal boutons. Here we summarize and briefly discuss these two classes of glutamatergic input and how their unique features, including their terminal bouton size and anatomy, are related to their suggested function.
 |
S. Murray Sherman is a Maurice Goldblatt Professor and Chairman of the Department of Neurobiology at the University of Chicago. He obtained his PhD in 1969 from the University of Pennsylvania under the supervision of James Sprague. As a postdoc he studied at the Australian National University under Peter Bishop. Sherman has had a long-standing interest in thalamic and cortical functional organization as well as in thalamocortical relationships. Iraklis Petrof has been working as a postdoctoral research associate at the Sherman laboratory since completing his PhD at the University of St Andrews in 2007. His main research objective is to examine the synaptic properties of glutamatergic pathways participating in thalamic and cortical circuits.
Not all glutamatergic pathways are equal
Following its arrival from the periphery, sensory information is fed forward and back between thalamus and cortex, but also within cortex itself, by a number of glutamatergic pathways. These glutamatergic pathways, however, are not homogeneous, and marked differences have been observed in their anatomy, synaptic properties and, as a consequence, their function. Work by our laboratory and others has shown that, based on these differences, glutamatergic pathways can be placed into one of two broad categories, Class 1 and Class 2.
Class 1 inputs
Differences in the synaptic properties of the two classes of input have been examined primarily in vitro, where activation of various glutamatergic pathways has produced dramatically different postsynaptic effects. For instance, >10 Hz electrical stimulation of Class 1 inputs produces large-amplitude, all-or-none, excitatory postsynaptic potentials (EPSPs) exhibiting paired-pulse depression (Fig. 1Aa), and these inputs activate ionotropic but not metabotropic glutamate receptors postsynaptically (Fig. 1Ac). Typical examples of Class 1 inputs include pathways that convey sensory information from the periphery to thalamus (these pathways are often collectively referred to as ‘lemniscal’), such as the medial lemniscal input to the ventral posterior medial nucleus (VPM/L, Castro-Alamancos, 2002), the optic tract input to the lateral geniculate nucleus (LGN, Reichova & Sherman, 2004) or portions of the tectothalamic input to the ventral segment of the medial geniculate nucleus (MGNv, Bartlett & Smith, 2002; Lee & Sherman, 2010). Similarly, feedforward corticothalamic pathways originating in layer 5 (Li et al. 2003; Reichova & Sherman, 2004) possess characteristics that are highly similar to those of lemniscal Class 1 inputs to thalamus.
Figure 1. Examples of synaptic and anatomical characteristics of Class 1 and Class 2 inputs to layer 4 and layers 2/3 cells of the mouse primary auditory cortex (A1).
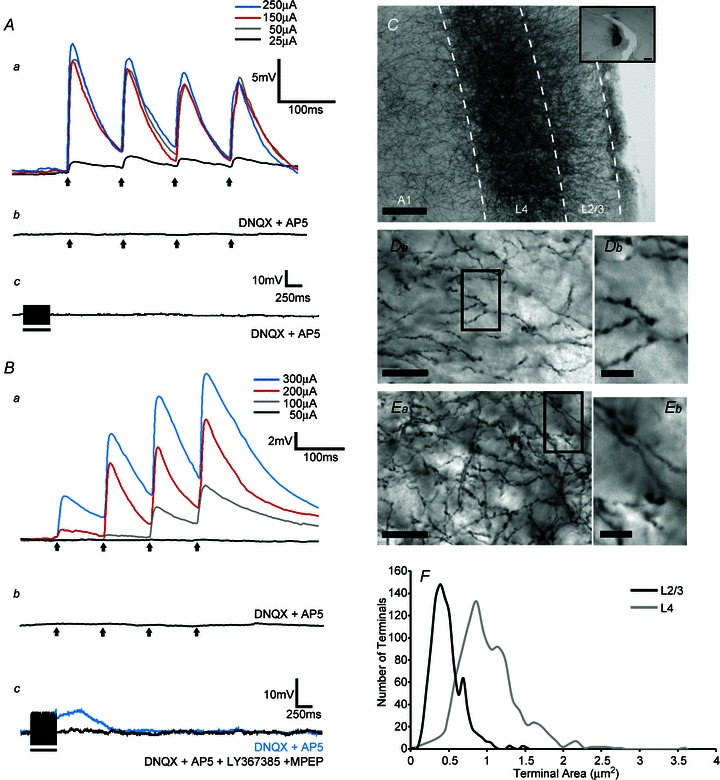
A, Class 1 response (average of 10 sweeps for each trace shown) in a layer 4 cell. Aa, this cell responded with paired-pulse depression to stimulation of ventral segment of the medial geniculate nucleus (MGNv) at 10 Hz. Responses were largely ‘all-or-none’, and EPSP amplitudes were largely unaffected by increases in stimulation intensities, reflecting the lack of convergence of MGNv inputs on layer 4 cells. Ab, stimulation at 10 Hz (250 μA), in the presence of the ionotropic glutamate antagonists DNQX and AP5 failed to produce any EPSPs. Ac, high frequency stimulation (125 Hz, 200 μA) in the presence of DNQX and AP5 did not produce any membrane potential changes, suggesting a lack of a metabotropic component. Arrows represent timing of stimulation for all 10 Hz trials and black bars represent the duration of stimulation in high frequency stimulation trials. B, Class 2 response in a layer 2/3 cell; also average of 10 sweeps for each trace. Ba, the cell responded with paired-pulse facilitation to MGNv stimulation at 10 Hz. Increasing stimulation intensities produced increases in EPSP amplitudes, possibly due to the high degree of convergence of these inputs onto a single cell. Bb, stimulation at 10 Hz (250 μA), in the presence of DNQX and AP5, failed to produce any EPSP. Bc, high frequency stimulation (125 Hz, 200 μA) in the presence of DNQX and AP5 produced a slow and prolonged membrane depolarization (blue trace) that could be blocked with a cocktail of type 1 (LY367385) and type 5 (2-methyl-6-(phenylethynyl)pyridine; MPEP) metabotropic glutamate receptor antagonists (black trace). C, anterograde labelling of axons and boutons in A1 following an injection of biotinylated dextran amine (BDA) in MGNv (inset). Da, BDA-labelled axons and boutons in layers 2/3 of A1. Highlighted area in Da can be seen at higher magnification in Db. Ea, BDA-labelled axons and boutons in layer 4 of A1. Highlighted area in Ea can be seen at higher magnification in Eb. F, histogram of bouton area in layers 2/3 and layer 4 of A1 (μm2). Scale bars: E, 125 mm; E inset, 0.25 mm; Da and Ea, 20 μm; Db and Eb, 5 μm). Reproduced from Viaene et al. (2011a).
Class 1 inputs have also been identified outside of thalamus, such as in certain thalamocortical pathways (Vieane et al. 2011a,b, see Fig. 1Aa), some inter-areal corticocortical pathways (Covic & Sherman, 2011; DePasquale & Sherman, 2011), and some local cortical inputs (DePasquale & Sherman, 2012). However, glutamatergic inputs in thalamocortical and intracortical circuitry are somewhat more variable in certain parameters than those described for thalamus, giving rise to three Class 1 subtypes.
Similar to lemniscal Class 1 inputs to thalamus, thalamocortical inputs to layer 4 and some inputs to layers 5 and 6 produce responses that are largely insensitive to increases in stimulation intensity following threshold (‘all-or-none’ responses), indicative of little or no axon convergence (Class 1A responses, Lee & Sherman, 2008; Viaene et al. 2011a,b,c). However, certain corticocortical Class 1 inputs (Covic & Sherman, 2011; DePasquale & Sherman, 2011) and some thalamocortical inputs to layers 5 and 6 (Viaene et al. 2011b) show a considerable degree of convergence, as suggested by the increased EPSP amplitudes following increases in stimulation intensity (Class 1B). Furthermore some thalamic projections to the subgranular cortical layers produce paired-pulse responses that resemble a mixture of facilitation and depression but do not activate metabotropic glutamate receptors and are thus functionally considered as Class 1 inputs (Class 1C, Viaene et al. 2011b).
Class 2 inputs
On the other hand, the synaptic properties of Class 2 inputs differ substantially from those of Class 1 inputs. For example, electrical stimulation of the local layer 6 to layer 4 pathway in the primary auditory and somatosensory cortices produces relatively small EPSPs, exhibiting paired-pulse facilitation (Lee & Sherman, 2008, 2009b). Similar short-term synaptic plasticity is also evident in other Class 2 pathways, such as the corticothalamic pathways arising in pyramidal cells of layer 6 (Bartlett & Smith, 2002; Li et al. 2003), some thalamocortical afferents (Viaene et al. 2011a,c; see Fig. 1Ba), some inter-areal corticocortical projections (Covic & Sherman, 2011; DePasquale & Sherman, 2011), as well as some intra-areal corticocortical pathways (Lee & Sherman, 2008, 2009b; DePasquale & Sherman, 2012). In addition to ionotropic glutamate receptors, these pathways are also capable of activating metabotropic glutamate receptors, both of Group I types leading to postsynaptic depolarization (Reichova & Sherman, 2004; Lee & Sherman, 2008; Covic & Sherman, 2011; Viaene et al. 2011a,c; see Fig. 1Bc) and also Group II types leading to hyperpolarization (Lee & Sherman, 2009a; DePasquale & Sherman, 2011). Finally Class 2 pathways are made up of axons with a much greater tendency to converge onto single cells compared to their Class 1 counterparts. This is evident by the monotonic or ‘graded’ fashion in which EPSPs increase in amplitude when stimulation intensity of these pathways is gradually increased (Fig. 1Ba), the result of the recruitment of a progressively larger number of converging axons.
Anatomical features of Class 1 and Class 2 inputs
In addition to the differences in their synaptic profile, Class 1 and Class 2 pathways have been associated with certain anatomical features. For instance, Class 1 pathways in thalamus are characterized by thick axons ending in dense terminal arbors that contain many relatively large (>2 μm2 in cross-sectional area) synaptic boutons. Examples of established Class 1 afferents, for which data about the size of their synaptic boutons is available, include the retinal input to LGN (Szentagothai, 1963; Colonnier & Guillery, 1964; Peters & Palay, 1966; Guillery, 1969; Hajdu et al. 1982; So et al. 1985; Sur et al. 1987; Van Horn et al. 2000; Li et al. 2003), the lemniscal input to VPM/L (Ralston, 1969), the inferior colliculus input to MGNv (Morest, 1975; Bartlett et al. 2000) and the corticothalamic pathways originating in layer 5 (Hoogland et al. 1991; Rouiller & Welker, 1991, 2000; Bourassa et al. 1995; Vidnyánszky et al. 1996; Feig & Harting, 1998; Li et al. 2003). A typical and rather interesting feature of thalamic Class 1 pathways is their tendency to contact large, presumably proximal dendrites (see Sherman & Guillery, 2006).
Similarly, cortical Class 1 pathways end in large terminal boutons. Examples of such pathways include a number of thalamocortical pathways (specifically, but not exclusively, those that terminate in layer 4 of cortex) in the auditory, visual and somatosensory systems (Ahmed et al. 1994; Lee & Sherman, 2008; Viaene et al. 2011a,c), and some corticocortical pathways (Covic & Sherman, 2011).
Unlike Class 1 inputs to thalamus, thalamic Class 2 inputs, such as those originating in corticothalamic afferents from layer 6 are made up of thin axons that terminate in small synaptic boutons (<1 μm2 in cross-sectional area) (Hoogland et al. 1991; Bartlett et al. 2000; Ichida & Casagrande, 2002; Li et al. 2003). These Class 2 inputs tend to contact their target postsynaptic cells on thinner, presumably distal dendrites (Sheman & Guillery, 2006). Cortical Class 2 inputs also terminate in small synaptic boutons. Examples include some corticocortical pathways (Covic & Sherman, 2011), and, as we reported recently, most thalamocortical inputs to layers 2/3 in the primary somatosensory (S1) and auditory (A1) cortices (Viaene et al. 2011a,c). It is also interesting that most of the projection from the posterior medial nucleus of the thalamus (POm) to all the layers of S1 is also Class 2 and is characterized by small terminals (Viaene et al. 2011c). The POm is considered a higher order thalamic nucleus for somatosensation, mostly involved in relaying information between cortical areas, and it projects mainly to higher order somatosensory cortical areas; its first order equivalent is VPM/L. Other first and higher order examples are, respectively, LGN and pulvinar for vision and the MGNv and MGNd for hearing; for review, see Sherman & Guillery, 2006.
Figure 1C shows anterograde labelling in the mouse primary auditory cortex (A1) following injections of biotinylated dextran amine (BDA) in the MGNv. Of particular interest is the comparison of terminal bouton sizes in layers 4 and 2/3. While thalamocortical boutons in layers 2/3, which deliver mainly Class 2 inputs, are relatively small, averaging less than 0.5 μm2 in cross-sectional area (Fig. 1Da, Db and F), the boutons in layer 4, which are associated with Class 1 inputs, are considerably larger, averaging more than 1 μm2 in cross-sectional area and in some cases as large as 2 μm2 or more (Fig. 1Ea, Eb and F). Note that, whereas the Class 1 input to layer 4 was characterized by large boutons, it also contained smaller boutons. It should be clarified that Class 1 and 2 inputs should not be confused with Guillery's (1966) definition of type I and type II axons. This appears to be a feature of other Class 1 pathways as well (Sur et al. 1987).
Mechanisms and functional implications
Table 1 summarizes the anatomical and synaptic differences between Class 1 and Class 2 glutamatergic inputs. Due to the large, purely ionotropic EPSPs that they produce, Class 1 inputs can exert strong effects on their postsynaptic targets that temporally match activity in the input, and the paired-pulse depression is plausibly an important property providing a gain control mechanism for synaptic processing (Chung et al. 2002), making them ideal for the reliable and faithful relay of information. Because of this, Class 1 inputs are often referred to as driver inputs, given that they are the main determinants of a postsynaptic cell's activity by virtue of defining its receptive field (Sherman & Guillery, 2006). Class 2 glutamatergic inputs on the other hand do not possess the required synaptic features for the effective relay of information. Instead, their relatively weak postsynaptic effects, extensive convergence, and their slow, prolonged metabotropic component are better suited for a modulatory role. For instance, the prolonged response is useful for the control of voltage- and time-gated conductances, and the response outlasts activity in the input by 100s of milliseconds to several seconds, features that are inconsistent with effective information flow. For this reason, Class 2 inputs are often referred to as modulators (Sherman & Guillery, 2006).
Table 1.
A summary of the anatomical and synaptic features of Class 1 and Class 2 inputs.
| Class 1 (driver) | Class 2 (modulator) | |
|---|---|---|
| Anatomical features | Large and small terminals | Small terminals |
| Contact proximal dendrites | Contact distant dendrites | |
| Thick axons | Thin axons | |
| Less convergence on target | More convergence on target | |
| Synaptic features | Large EPSPs | Small EPSPs |
| Paired-pulse depression | Paired-pulse facilitation | |
| Activate ionotropic glutamate receptors | Activate ionotropic and metabotropic glutamate receptors |
An interesting point that needs to be made is that even though Class 1 inputs are the main bearers of information in thalamic and cortical circuits, they are vastly outnumbered by Class 2 inputs, accounting for less than 10% of the total number of synapses in thalamus and cortex, with some estimates putting them as low as 2% (Wang et al. 2002; Van Horn & Sherman, 2004). Because in thalamus Class 1 terminals produce ∼10 synapses, and Class 2 rarely more than one, these synaptic ratios imply a much lower ratio of Class 1 to Class 2 terminal boutons (Van Horn et al. 2000.) Even though these numbers may somewhat underestimate the total number of Class 1 inputs, as they focus mainly on large boutons, it is evident that the stronger synaptic effects of Class 1 inputs are not due to a numerical superiority over those of Class 2.
Although glutamatergic pathways tested to date fall clearly into the Class 1 or 2 categories, more classes may well emerge with further testing of other brain circuits.
Figure 2 shows a diagrammatic representation of glutamatergic pathways in the visual, auditory and somatosensory systems, for which both terminal anatomy, especially with regard to bouton size, and synaptic properties are known. Even this overly simplified form reveals that a highly complex matrix of Class 1 and Class 2 input interactions occurs in thalamic and cortical circuits.
Figure 2. A diagrammatic representation of glutamatergic pathways in thalamic and cortical circuits for which both terminal anatomy, especially with regard to bouton size, and synaptic properties are known.
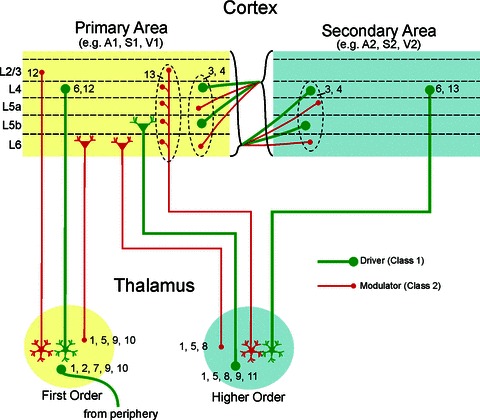
Numbers near boutons reflect literature references providing evidence for the classification of each input: (1) Bartlet et al. (2002); (2) Castro-Alamancos (2002); (3) Covic & Sherman (2011); (4) DePasquale & Sherman (2011); (5) Hoogland et al. (1991); (6) Lee & Sherman (2008); (7) Lee & Sherman (2010); (8) Li et al. (2003); (9) Reichova & Sherman (2004); (10) Van Horn et al. (2000); (11) Van Horn & Sherman (2004); (12) Viaene et al. (2011a); (13) Viaene et al. (2011c).
An interesting question that has not been addressed is whether the large and small terminals of Class 1 pathways originate from the same cells. Even though some evidence suggests that this may be indeed the case (Sur et al. 1987; Tamamaki et al. 1995) the exact functional roles of small versus large boutons of Class 1 inputs remains unknown. Similarly it is unknown whether synapses from separate branches of a single axon can possess different synaptic properties (Class 1 vs. Class 2) or not (e.g. Reyes et al. 1998). Answering these questions will provide us with a better understanding of the exact mechanisms behind the function of Class 1 and 2 glutamatergic inputs.
Conclusions
Even though a great number of questions remain about the mechanisms of glutamatergic synaptic transmission, there is considerable evidence to suggest that certain anatomical and functional features of inputs are correlated. Glutamatergic pathways that terminate in large boutons appear to possess properties that enable them to exert strong postsynaptic effects and to be the driving force behind the transmission of information. The postsynaptic effects of pathways associated with small terminal boutons, on the other hand, are substantially more subtle and modulatory, their role largely being to control various aspects of how Class 1 inputs are processed. Nonetheless, given that many glutamatergic pathways have remained unexplored with regard to their synaptic properties, parsimony dictates that their identification as Class 1 or 2 should not be assumed purely on their anatomical features, or vice versa. As the number of glutamatergic pathways with known synaptic profiles grows, so does our understanding of the brain circuits and the mechanisms behind their function.
References
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience. 2002;113:957–974. doi: 10.1016/s0306-4522(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Stark JM, Guillery RW, Smith PH. Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body. Neuroscience. 2000;100:811–828. doi: 10.1016/s0306-4522(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol. 2002;87:946–953. doi: 10.1152/jn.00426.2001. [DOI] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Colonnier M, Guillery RW. Synaptic organization in the lateral geniculate nucleus of the monkey. Z Zellforsch Mikrosk Anat. 1964;62:333–355. doi: 10.1007/BF00339284. [DOI] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale R, Sherman SM. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J Neurosci. 2011;31:16494–16506. doi: 10.1523/JNEUROSCI.3664-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale R, Sherman SM. Modulatory effects of metabotropic glutamate receptors on local cortical circuits. J Neurosci. 2012;32:7364–7372. doi: 10.1523/JNEUROSCI.0090-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig S, Harting JK. Corticocortical communication via the thalamus: ultrastructural studies of corticothalamic projections from area 17 to the lateral posterior nucleus of the cat and inferior pulvinar nucleus of the owl monkey. J Comp Neurol. 1998;395:281–295. doi: 10.1002/(sici)1096-9861(19980808)395:3<281::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Guillery RW. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966;128:21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat. 1969;96:1–38. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- Hajdu F, Hassler R, Somogyi G. Neuronal and synaptic organization of the lateral geniculate nucleus of the tree shrew, Tupaia glis. Cell Tissue Res. 1982;224:207–223. doi: 10.1007/BF00217280. [DOI] [PubMed] [Google Scholar]
- Hoogland PV, Wouterlood FG, Welker E, Van der Loos H. Ultrastructure of giant and small thalamic terminals of cortical origin: a study of the projections from the barrel cortex in mice using Phaseolus vulgaris leuco-agglutinin (PHA-L) Exp Brain Res. 1991;87:159–172. doi: 10.1007/BF00228517. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Casagrande VA. Organization of the feedback pathway from striate cortex (V1) to the lateral geniculate nucleus (LGN) in the owl monkey (Aotus trivirgatus. J Comp Neurol. 2002;454:272–283. doi: 10.1002/cne.10441. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009a;19:2281–2289. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Modulator property of the intrinsic cortical projection from layer 6 to layer 4. Front Syst Neurosci. 2009b;3:1–5. doi: 10.3389/neuro.06.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc Natl Acad Sci U S A. 2010;107:372–377. doi: 10.1073/pnas.0907873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- Morest DK. Synaptic relationships of Golgi type II cells in the medial geniculate body of the cat. J Comp Neurol. 1975;162:157–193. doi: 10.1002/cne.901620202. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL. The morphology of laminae A and A1 of the dorsal nucleus of the lateral geniculate body of the cat. J Anat. 1966;100:451–486. [PMC free article] [PubMed] [Google Scholar]
- Ralston HJ. The synaptic organization of lemniscal projections to the ventrobasal thalamus of the cat. Brain Res. 1969;14:99–115. doi: 10.1016/0006-8993(69)90033-x. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res. 1991;56:179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. Cambridge, MA, USA: MIT Press; 2006. [Google Scholar]
- So KF, Campbell G, Lieberman AR. Synaptic organization of the dorsal lateral geniculate nucleus in the adult hamster. An electron microscope study using degeneration and horseradish peroxidase tracing techniques. Anat Embryol (Berl) 1985;171:223–234. doi: 10.1007/BF00341417. [DOI] [PubMed] [Google Scholar]
- Sur M, Esguerra M, Garraghty PE, Kritzer MF, Sherman SM. Morphology of physiologically identified retinogeniculate X- and Y-axons in the cat. J Neurophysiol. 1987;58:1–32. doi: 10.1152/jn.1987.58.1.1. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. The structure of the synapse in the lateral geniculate body. Acta Anat (Basel) 1963;55:166–185. [PubMed] [Google Scholar]
- Tamamaki N, Uhlrich DJ, Sherman SM. Morphology of physiologically identified retinal X and Y axons in the cat's thalamus and midbrain as revealed by intraaxonal injection of biocytin. J Comp Neurol. 1995;354:583–607. doi: 10.1002/cne.903540408. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Erişir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. J Comp Neurol. 2000;416:509–520. [PubMed] [Google Scholar]
- Van Horn SC, Sherman SM. Differences in projection patterns between large and small corticothalamic terminals. J Comp Neurol. 2004;475:406–415. doi: 10.1002/cne.20187. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. J Neurophysiol. 2011a;105:279–292. doi: 10.1152/jn.00747.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci. 2011b;31:12738–12747. doi: 10.1523/JNEUROSCI.1565-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci U S A. 2011c;108:18156–18161. doi: 10.1073/pnas.1114828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidnyánszky Z, Borostyánkõi Z, Görcs TJ, Hámori J. Light and electron microscopic analysis of synaptic input from cortical area 17 to the lateral posterior nucleus in cats. Exp Brain Res. 1996;109:63–70. doi: 10.1007/BF00228627. [DOI] [PubMed] [Google Scholar]
- Wang S, Eisenback MA, Bickford ME. Relative distribution of synapses in the pulvinar nucleus of the cat: implications regarding the “driver/modulator” theory of thalamic function. J Comp Neurol. 2002;454:482–494. doi: 10.1002/cne.10453. [DOI] [PubMed] [Google Scholar]


