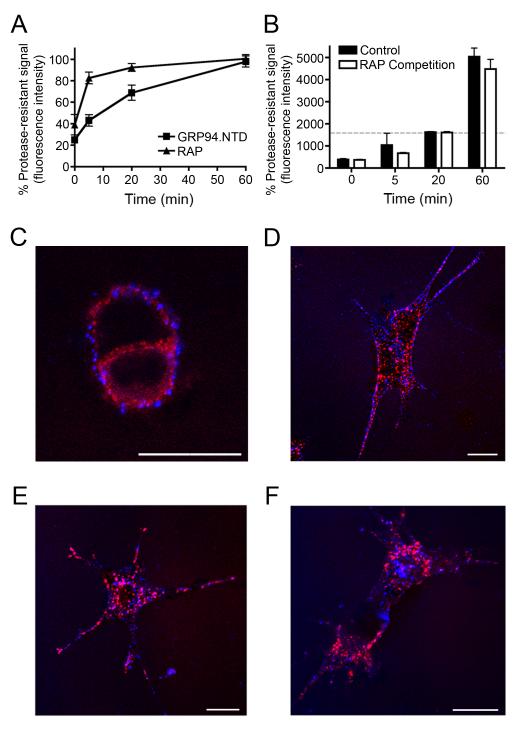
Figure 6. GRP94.NTD and RAP are internalized via spatially and kinetically distinct pathways.
A & B. MEF-1 cells were incubated with either 10 μg/mL RAP or 25 μg/mL GRP94.NTD, washed, warmed to 37°C for 0, 5, 20, or 60 min, and placed on ice to arrest endocytosis. Residual surface-bound ligands were removed by proteolysis on ice. The percent protease-resistant signal was calculated by normalizing the protease resistant signal to the total signal at each time point. Results are expressed as the mean of three independent experiments ± SD B. Comparison of MEF-1 cells treated either as in A (control) or treated with 10 μg/mL RAP prior to GRP94.NTD incubation and excess RAP during internalization (RAP Competition). Residual surface-bound ligands were removed by proteolysis on ice. The dashed gray bar indicates total GRP94.NTD surface binding prior to treatment with extracellular protease. C-F. MEF-1 cells were incubated with 5 μg/mL RAP (red) and 40 μg/mL GRP94.NTD (blue), washed, and warmed to 37°C for 0 (C), 5 (D), 15 (E), or 60 (F) min to allow internalization. Cells were then fixed and processed for confocal microscopy. Scale bars: 20 μm. All data presented are representative of three independent replicates.