Abstract
This study compared patterns of prenatal care among mothers who used methamphetamine (MA) during pregnancy and non-using mothers in the US and New Zealand (NZ), and evaluated associations among maternal drug use, child protective services (CPS) referral, and inadequate prenatal care in both countries. The sample consisted of 182 mothers in the MA-Exposed and 196 in the Comparison groups in the US, and 107 mothers in the MA-Exposed and 112 in the Comparison groups in NZ. Positive toxicology results and/or maternal report of MA use during pregnancy were used to identify MA use. Information about sociodemographics, prenatal care and prenatal substance use was collected by maternal interview. MA-use during pregnancy is associated with lower socio-economic status, single marital status, and CPS referral in both NZ and the US. Compared to their non-using counterparts, MA-using mothers in the US had significantly higher rates of inadequate prenatal care. No association was found between inadequate care and MA-use in NZ. In the US, inadequate prenatal care was associated with CPS referral, but not in NZ. Referral to CPS for drug use only composed 40 % of all referrals in the US, but only 15 % of referrals in NZ. In our study population, prenatal MA-use and CPS referral eclipse maternal sociodemographics in explanatory power for inadequate prenatal care. The predominant effect of CPS referral in the US is especially interesting, and should encourage further research on whether the US policy of mandatory reporting discourages drug-using mothers from seeking antenatal care.
Keywords: Methamphetamine, Adequate prenatal care, New Zealand, Kessner Index, Child protective services
Introduction
Methamphetamine (MA) use is a growing worldwide public health issue [1–3], with dramatic increases in the Asia-Pacific region [4, 5]. Of particular concern is MA use during pregnancy [6]: the Infant Development, Environment, and Lifestyle (IDEAL) study estimated that 5.5 % of the pregnant women in its study sites used MA [7]. While no similar specific estimates are available in New Zealand (NZ), NZ’s statistics on MA use parallel trends worldwide [8, 9], with a fivefold increase in the past 7 years amongst pregnant women in Auckland [10].
A primary worry concerning MA use during pregnancy is its association with inadequate prenatal care. Research demonstrates a clear correlation between drug use during pregnancy and inadequate prenatal care or late access to care [11, 12], both of which are linked to maternal and neonatal risks including a lack of breastfeeding, postnatal care, well-child visits, child immunizations, and an increased likelihood of loss of custody [13]. For drug-using women, prenatal care is especially beneficial as it facilitates drug-use monitoring and linkages with mental health, nutritional and educational services, while identifying psychological and social issues. Adequate prenatal care among cocaine-using women is associated with positive perinatal outcomes, including greater birthweight and head circumference [14–20], decreased prematurity [15–17] and a lower likelihood of having infants born small for gestational age [15, 16].
However, perceived and real barriers prevent drug-using women from obtaining early and consistent care throughout pregnancy, including financial and drug-related social problems. One of the most consistently reported obstacles is the belief that accessing prenatal care will lead to reports to legal or child protection agencies [21–29]. This belief among pregnant drug users in the US may be attributable to the legally-mandated requirement for health professionals to report maternal drug use to child protection services [30].
The majority of studies examining substance abuse and prenatal care have been conducted in the US, and the extent to which findings can be generalized to countries with different medical and legal systems is unknown. To date, no studies have compared use of prenatal care among drug-using women in countries with differing legal and social policies. Given its importance, it is useful to understand the correlates of adequate prenatal care and the possible impact of legal, social and/or healthcare policies on access to and usage of prenatal care.
We chose to examine patterns of prenatal care in NZ and the US: despite the cultural similarities between the two countries, there are three notable differences in the management of maternity care that potentially affect utilization of care among drug-using women. First, in NZ, no legal mandate exists to report a woman to legal or child protection services upon revealing drug use during pregnancy. Second, pre- and post-natal care is free for NZ residents under the country’s Universal Healthcare system. Finally, most women receive prenatal care throughout their pregnancy, at birth and post-natally, from midwives, promoting continuity of care and stronger provider-patient relationships.
Our study uses data from the IDEAL study, a longitudinal study of prenatal MA exposure and child outcomes, to compare patterns of prenatal care among MA-using and non-using mothers in the US and NZ. We also evaluated associations between prenatal MA use, sociodemographic status, CPS referral, and adequate prenatal care in these countries.
Methods
Overview
The IDEAL study involved four US sites (Los Angeles, CA; Des Moines, IA; Tulsa, OK; and Honolulu, HI) and one international site (Auckland, NZ). Due to differing site regulations and methods of delivering prenatal care between the two countries, recruitment procedures slightly varied.
In the US, study protocol was reviewed and approved by each site’s IRB. A federal Certificate of Confidentiality (COC) was obtained to assure confidentiality regarding sensitive information about substance abuse, superseding mandatory reporting of illegal drug use but not evidence of abuse and neglect. At all four sites, staff members were responsible for monitoring hospital delivery logs and attempted to approach every mother who delivered an infant within the last 48 h, prior to discharge. The purpose and scope of the study were explained, along with assurances afforded by the COC. If the mother signed informed consent to participate, the staff member administered the Lifestyle Interview and collected infant meconium.
In NZ, approval was granted by both the Auckland and Waitemata District Health Boards (DHBs), and finalized by the NZ Ministry of Health’s Northern Regional Ethics Committee. Because of the inclusion of Maori participants, the study consulted local Iwi and Maori health care agencies before obtaining approval from the Maori Research Committees of the Auckland and Waitemata DHBs. While mothers were again ensured confidentiality, a COC was not required because NZ has no analogous policy of mandatory reporting.
Recruitment in NZ took place during pregnancy. In NZ, prenatal care is usually provided by midwives; thus, in NZ, midwives in the Auckland region were requested to refer potential subjects. All referred mothers met with study staff and, if interested, provided written consent for participation. Staff met again with mothers immediately after delivery, and prior to discharge, administered the Lifestyle Interview and collected infant meconium.
In both countries, maternal exclusion criteria were: LSD, PCP and/or other hallucinogen use during the pregnancy; younger than 18 (US) and 17.5 (NZ) years of age; history of hospitalization for intellectual disability or emotional disorders; low cognitive functioning; overt psychotic behavior or documented psychosis; and an inability to speak English (except Maori in NZ). Infant exclusion criteria were: critical illness at birth/unlikely to survive; multiple births; major life-threatening congenital anomaly; documented chromosomal abnormality associated with mental or neurologic deficiency; overt TORCH infection; and/or sibling previously enrolled in the study.
Participants
A case–control design was used: mothers and their infants were classified into either the ‘Exposed group’ by maternal report of MA use during pregnancy and/or positive toxicology results from meconium screening or into the ‘Comparison group’ by negative maternal-report and meconium screen. The two groups were matched within site in the US and within NZ by race/ethnicity, infant birth weight category (<1,500 g, 1,500–2,500 g,>2,500 g), and educational level. In the US, mothers were matched on private versus public insurance, which was not applicable in NZ.
The direct-recruiting method in the US allowed most participants to be matched one-to-one. When characteristics were difficult to match (e.g., Asian, >2,500 g, public insurance, high school not completed), a few Comparison group participants were enrolled prior to corresponding Exposed participants, leading to uneven group sizes. In NZ, the midwife-referral method of recruiting narrowed the pool of potential participants; therefore, group level matching was conducted. The final sample sizes were 182 Exposed and 196 Comparison participants in the US, and 107 and 112 participants in NZ, respectively. Details on recruitment protocols and response rates are provided in earlier publications [1, 31].
Measures
All study instruments were administered by trained staff members. The Lifestyle Interview collected details about the pregnancy and sociodemographics: educational level, age, race/ethnicity, partner status, insurance type (US only), and socioeconomic status (SES), which was calculated using the four-factor Hollingshead Index (Group 5 ═ low SES). Race/ethnicity was dichotomized into ‘minority’ (all non-white participants) versus ‘non-minority’ (participants of white/European-ancestry) status.
The Lifestyle Interview asked about referrals made to Child, Youth, and Family Services (CYFS; NZ) and Child Protective Services (CPS; US). Referrals to CPS were also obtained from the participants’ medical charts. Hospital records included a section on the social conditions of the infant’s discharge. Reasons for CPS referral included: (1) In utero drug exposure and/or maternal drug or alcohol use; (2) Abandonment by mother; (3) Mother thought to be incapable of caring for child; (4) Evidence of neglect; (5) Evidence of physical and/or sexual abuse; (6) Mother’s social or economic circumstances; (7) Mother’s physical or mental condition; (8) Mother already known to CYFS; (9) Mother incarcerated; and (10) Mother deceased. The information was coded as either a yes or no ‘CPS referral’. Because it was possible to be simultaneously referred for both drug-related (#1) and non-drug/other related (#2–10) reasons, CPS referral reasons were categorized as ‘Drug Only’, ‘Both Drug/Other’ and ‘Other Only’. There were too few cases of ‘Other Only’ (N ═ 3 [1.6 %] in the US, N ═ 1 [0.9 %] in NZ) to analyze as a distinct group.
Inadequate prenatal care, measured using the Kessner Index [32], was derived from questions in the Lifestyle Interview, specifically the number of prenatal visits and the GA at first prenatal visit. The Kessner Index ranks the adequacy of prenatal care into three categories: adequate, intermediate, and inadequate. We created a binary measure of either ‘inadequate’ or ‘intermediate/adequate’ prenatal care. All cases with no prenatal visits (N ═ 12 in the US, N ═ 0 in NZ) were classified as inadequate care. This modified Kessner scale does not take into consideration prenatal service quality.
The Substance Use Inventory (SUI) assessed maternal substance use during pregnancy. Two variables were measured: (1) a dichotomous variable denoting ‘any’ use of tobacco, alcohol, marijuana, methamphetamine; and (2) continuous measures of drug use quantity per day: tobacco in number of cigarettes; alcohol in ounces; and marijuana in joints. For alcohol, standard drinks were converted to absolute alcohol ounces based on each country’s conventions.
Statistical Analysis
All statistical analyses were conducted using SPSS version 17.0. Analysis of variance (ANOVA) and Chi-square statistics were used to compare groups within and across each country on maternal demographic characteristics, prenatal use of tobacco, alcohol and marijuana, prenatal care and its components (Table 1), and patterns of CPS/CYFS referral (Table 3). Mann–Whitney tests were used to compare tobacco, alcohol, and marijuana use quantities within and across country and exposure groups (Table 1).
Table 1.
A comparison of maternal sociodemographic factors, prenatal care utilization, and maternal prenatal drug use in the Methamphetamine (MA) Exposed and Comparison groups in the US and NZ, and a direct comparison of the MA Exposed cohorts in the US (US-MA) and NZ (NZ-MA)
| US |
NZ |
P values |
|||||
|---|---|---|---|---|---|---|---|
| Exposed (N═182) |
Comparison (N═196) |
Exposed (N ═ 107) |
Comparison (N ═ 112) |
US Exposed vs. comparison |
NZ Exposed vs. comparison |
US-MA vs. NZ-MA |
|
| N (%) or Mean (SD) | P | P | P | ||||
| Sociodemographics | |||||||
| Race/ethnicity (% minority) | 116 (63.7 %) | 119 (60.7 %) | 47 (43.9 %) | 60 (53.6 %) | 0.545 | 0.153 | 0.001 |
| SES | 24.9 (9.2)a | 30.4 (9.6)b | 21.9 (9.8)c | 29.6 (13.0) | <0.001 | <0.001 | 0.010 |
| Uninsured | 4 (2.2 %) | 2 (1.0 %) | n/a | n/a | 0.434 | – | – |
| No partner | 99 (54.4 %) | 67 (34.2 %) | 55 (51.4) | 29 (25.9) | <0.001 | <0.001 | 0.622 |
| Educational level (<high school/<5th form) | 84 (46.4 %)a | 77 (39.5 %)b | 66 (62.9 %)d | 56 (50.0 %) | 0.175 | 0.056 | 0.007 |
| Maternal age (years) | 25.9 (5.7) | 24.22 (5.3) | 26.61 (6.1) | 25.31 (6.7) | 0.003 | 0.137 | 0.317 |
| Child protective services referral | 112 (61.5 %) | 8 (4.1 %) | 39 (36.4 %) | 3 (2.7 %) | <0.001 | <0.001 | <0.001 |
| Prenatal care | |||||||
| Number of prenatal visits | 11.4 (7.4)e | 14.35 (5.4)f | 15.79 (7.0) | 17.02 (5.8) | <0.001 | 0.156 | <0.001 |
| Gestational age at 1st prenatal visit (weeks) | 14.75 (8.1)g | 9.48 (5.6)h | 15.94 (6.8)d | 13.28 (5.6)c | <0.001 | 0.002 | 0.206 |
| Inadequate prenatal care | 42 (23.1 %) | 9 (4.6 %) | 9 (8.4 %) | 4 (3.6 %) | <0.001 | 0.130 | 0.002 |
| Prenatal drug use | |||||||
| Tobacco | 145 (79.7 %) | 52 (26.5 %) | 93 (87.0 %) | 60 (53.6 %) | <0.001 | <0.001 | 0.119 |
| Alcohol | 71 (39.0 %) | 25 (12.8 %) | 68 (63.6 %) | 63 (56.3 %) | <0.001 | 0.271 | <0.001 |
| Marijuana | 64 (35.2 %) | 7 (3.6 %) | 67 (62.6 %) | 24 (21.4 %) | <0.001 | <0.001 | <0.001 |
| Cigarettes/day | 7.00 (8.3)a | 1.62 (4.5) | 8.37 (7.3) | 3.36 (5.5) | <0.001 | <0.001 | 0.018 |
| Ounces alcohol/day | 0.13 (0.5)a | 0.003 (0.02) | 0.32 (0.8) | 0.12 (0.3) | <0.001 | 0.171 | <0.001 |
| Joints/day | 0.10 (0.3)i | 0.01 (0.09) | 0.46 (1.0) | 0.18 (0.7) | <0.001 | <0.001 | <0.001 |
Sample sizes vary slightly due to missing data N ═ 181
N ═ 195
N ═ 106
N ═ 105
N ═ 174
N ═ 192
N ═ 173
N ═ 188
N ═ 180
Bold values indicate significant findings (P < 0.05)
Table 3.
Patterns of CPS referral and out-of-home foster placement in only the MA exposed groups in the US and NZ
| US-MA (N ═ 182) N (%) |
NZ-MA (N ═ 107) N (%) |
P value | |
|---|---|---|---|
| CPS referrals | 112 (61.50 %) | 39 (36.40 %) | <0.001 |
| Out of home placement | 57 (31.8 %)a | 6 (5.6 %) | <0.001 |
| Drug only referrals | 44 (24.2 %) | 6 (5.6 %) | <0.001 |
| Both drug/other referrals | 65 (35.7 %) | 32 (29.9 %) | 0.313 |
N ═ 179
Bold values indicate significant findings (P < 0.05)
All variables in Table 1 were examined for possible inclusion as potential factors related to inadequate prenatal care. Factors were selected based on conceptual reasons, previous literature, and characteristics that differed between exposure groups in either country. The final chosen variables were: MA-exposure, quantities of tobacco, alcohol, and marijuana use, minority status, SES, maternal age, partner status, and CPS referral. Maternal education was highly correlated with SES and excluded. Tobacco, alcohol, and marijuana quantities, maternal age and SES were analyzed as continuous measures while minority status, partner status, and CPS referral were analyzed as binary variables.
For each country, two logistic regressions were conducted, testing the effects of (1) MA-exposure and tobacco, alcohol, and marijuana quantities; and (2) MA-exposure, tobacco, alcohol, and marijuana quantities, minority status, SES, maternal age, partner status, and CPS referral (Table 2) on inadequate care in each country. Because the US cohort was matched, the 4-level site effect was not tested. Statistical significance was accepted at P < 0.05.
Table 2.
Two logistic regressions modeling predictors of inadequate prenatal care, entering MA exposure and continuous measures of other drug use (model 1) and MA exposure, continuous measures of additional substance use, and maternal sociodemographic factors as predictors (model 2)
| Predictors | US |
NZ |
||||
|---|---|---|---|---|---|---|
| P value | Odds ratio | 95th C.I. | P value | Odds ratio | 95th C.I. | |
| Model 1: substance use variables | ||||||
| Methamphetamine exposure | <0.001 | 4.63 | 2.08–10.32 | 0.345 | 1.87 | 0.51–6.87 |
| # Of cigarettes per day-renatal | 0.064 | 1.04 | 0.10–1.08 | 0.637 | 1.02 | 0.94–1.11 |
| oz. Of absolute alcohol per day-prenatal | 0.157 | 2.00 | 0.77–5.25 | 0.529 | 0.69 | 0.22–2.18 |
| # Of joints per day- prenatal | 0.820 | 0.85 | 0.22–3.37 | 0.191 | 1.48 | 0.82–2.65 |
| Model 2: substance use variables and maternal sociodemographics | ||||||
| Methamphetamine exposure | 0.600 | 1.31 | 0.48–3.60 | 0.521 | 1.65 | 0.36–7.55 |
| # Of cigarettes per day-prenatal | 0.390 | 1.02 | 0.98–1.07 | 0.860 | 0.99 | 0.90–1.09 |
| oz. Of absolute alcohol per day-prenatal | 0.277 | 1.69 | 0.66–4.37 | 0.424 | 0.64 | 0.21–1.93 |
| # Of joints per day-prenatal | 0.501 | 1.70 | 0.36–7.90 | 0.413 | 1.30 | 0.69–2.45 |
| Minority status | 0.944 | 0.97 | 0.44–2.17 | 0.166 | 2.73 | 0.66–11.31 |
| SES | 0.339 | 0.98 | 0.94–1.02 | 0.062 | 0.90 | 0.81–1.01 |
| Maternal age (years) | 0.472 | 0.98 | 0.92–1.04 | 0.453 | 1.04 | 0.94–1.14 |
| No partner | 0.195 | 1.60 | 0.79–3.18 | 0.871 | 1.12 | 0.28–4.43 |
| Child protective services referral | <0.001 | 7.15 | 2.75–18.60 | 0.311 | 0.40 | 0.07–2.37 |
Bold values indicate significant findings (P <0.05)
Results
Maternal Demographics
In both the US and NZ, the Exposed group was significantly less likely to have a partner, more likely to be of lower SES and have a referral to CPS compared to the Comparison group (Table 1). A greater percentage of MA-using mothers in the US (US-MA) were minorities and had a referral to CPS compared to MA-using mothers in NZ (NZ-MA). The NZ-MA cohort was more likely to be of lower SES and educational attainment than the US-MA cohort (Table 1).
Prenatal Care
In the US, the Exposed group had significantly fewer prenatal visits, attended their first prenatal appointment later, and had a higher rate of inadequate prenatal care compared to the Comparison group. In contrast, in NZ, the only significant difference between the two groups was the relative lateness of the first prenatal visit for the Exposed group (Table 1).
A comparison of the US-MA and NZ-MA groups revealed that the US-MA cohort had fewer prenatal visits and were more likely to receive inadequate prenatal care. No significant difference existed in the GA at first prenatal visit (Table 1).
Prenatal Substance Use
In the US, the MA group was more likely than their nonusing counterparts to use tobacco, alcohol and marijuana and to use these substances in greater quantities. Identical trends were found in NZ, except that no significant differences existed between the NZ Exposed and Comparison groups in terms of prevalence or quantity of alcohol use during pregnancy (Table 1).
Comparing the NZ-MA and US-MA groups directly, a larger percentage of NZ-MA participants used alcohol and marijuana and consumed greater amounts of tobacco, alcohol, and marijuana than the US-MA group. Tobacco use was not significantly different between the groups (Table 1).
Correlates of Inadequate Care
MA-exposure was significantly associated with inadequate prenatal care in the US (P < 0.001), with mothers in the Exposed group being 4.63 times more likely to receive inadequate care than their matched Comparisons. In contrast, this association was not found in the NZ cohort (Table 2). Interestingly, after accounting for demographic characteristics and prenatal drug use, the only significant correlate of inadequate prenatal care in the US was CPS referral (P < 0.001), with referred mothers being 7.15 times as likely to receive inadequate care. In the NZ cohort, none of the variables were significantly associated with inadequate prenatal care (Table 2).
Reasons for CPS Referral
A significantly larger rate of CPS referral and subsequent out-of-home placements were observed in the US-MA group compared to the NZ-MA group, consistent with the US legal mandate to report drug use during pregnancy (Table 3). While the US-MA cohort has a significantly higher percentage of Drug Only CPS referrals relative to the NZ-MA cohort, a Chi-square test comparing the NZ-MA and US-MA groups found no significant difference in the percentage of ‘Both Drug/Other’ CPS referrals between the two cohorts (Table 3).
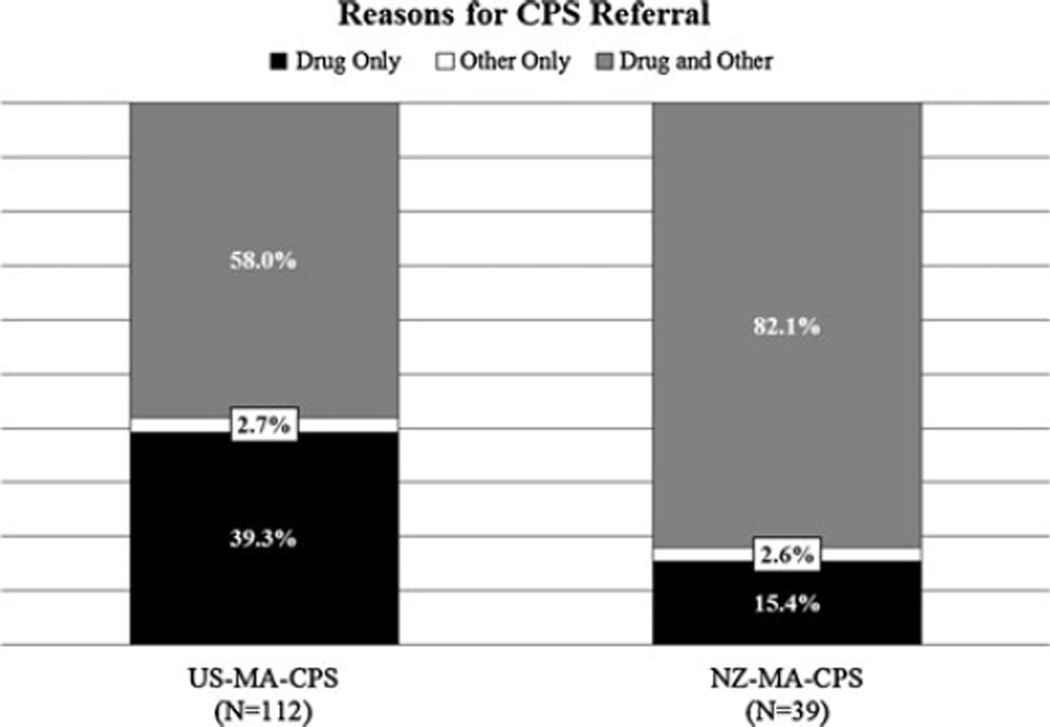
Focusing on MA-using mothers who were reported to CPS (hereby designated NZ-MA-CPS and US-MA-CPS), we found that patterns of CPS referral were consistent with the reporting practices of each country (Fig. 1). A significantly greater proportion (P < 0.001) of the US-MA-CPS group (39.3 %) were reported for ‘Drug Only’ reasons compared to the NZ-MA-CPS group (15.4 %), again reflecting the American policy of mandatory reporting of prenatal drug use to CPS by state statutes. All four US sites have active reporting statutes to CPS or other agencies. NZ has no such policy. A significantly greater proportion (P ═ 0.007) of the NZ-MA-CPS group (82.1 %) were reported for ‘Both Drug/Other’ reasons compared to the US-MA-CPS group (58.0 %). We hypothesize that this disparity is due again to the differing reporting practices in the two countries: in NZ, CPS referrals are made only with the co-occurence of other adverse environmental conditions.
Fig. 1.
Comparison of reasons for referral to Child Protective Services among the MA Exposed cohorts in the US and NZ. For each country, the percentages are taken out of the total population of MA-using mothers with a history of CPS referral (designated as US-MA-CPS and NZ-MA-CPS). Compared to NZ, the US-MA-CPS cohort had a significantly higher proportion of ‘Drug Only’ referrals (39.3 vs. 15.4 %, P < 0.001). Similarly, relative to its US comparison cohort, the NZ-MA-CPS group had a significantly higher proportion of ‘Both Drug/Other’ referrals. (82.1 vs. 58.0 %, P ═ 0.007; data not shown). These patterns reflect the stricter policies regarding maternal substance use and CPS involvement of the US
Discussion
This study found that MA use during pregnancy is associated with lower SES, single marital status, and referral to CPS in both NZ and the US, as reported by earlier studies. Compared to their non-using counterparts, the US-MA group had significantly higher rates of inadequate prenatal care, corroborating the results of previous studies [11, 12, 33–41]; no association was found between inadequate prenatal care and MA use in NZ. The only variable associated with inadequate prenatal care in the US that retained statistical significance was CPS referral. In NZ, none of the studied variables were significantly correlated with inadequate care.
Comparing MA users between countries, the NZ-MA group was more likely to have lower SES and educational attainment, while the US-MA group was more likely to be of minority status and have a CPS referral. There was also a much higher rate of CPS referral and subsequent out-of-home placement in the US-MA group. Further analysis revealed that a much larger percentage of the US-MA-CPS cohort was reported for ‘Drug Only’ reasons in contrast to the more predominant ‘Both Drug/Other’ reasons for CPS referral in the NZ-MA-CPS cohort. These results are consistent with the current US policy of mandatory reporting.
The major findings of this study are the country-dependent disparity in rates of adequate care among MA-using mothers and the correlates of inadequate care in both NZ and the US. While previous studies based in the US have identified maternal drug use as a significant barrier for prenatal care [11, 12, 34–41], to date, no studies have directly compared the impact of maternal drug use on adequacy of prenatal care in two different countries. This study is unique in that it not only examines the role of MA-exposure in prenatal care in the context of two countries with dissimilar medical and legal systems, but also examines a variety of socioeconomic and demographic factors as potential correlates of inadequate care.
Of immediate interest, given the higher incidence of inadequate prenatal care among the MA-using mothers in the US, is the potential role of health insurance: previous studies in the US have emphasized the importance of health insurance as a determinant of adequate prenatal care, with lack of insurance presenting a severe financial deterrent to prenatal care utilization [26, 33, 36, 37, 39, 42–50]. Comparative studies of the US and European countries have suggested that the greater inaccessibility of American prenatal healthcare is due, to a large degree, to the incomplete financial coverage provided by the US’s private insurance systems [51–57].
We examined whether the difference in adequate care between the two countries was attributable to structural healthcare system differences, with NZ’s Universal Healthcare removing financial barriers to improve access to prenatal care. We hypothesized that a greater proportion of MA-using mothers in the US might not have been able to access adequate care due to a lack of health insurance coverage and subsequent financial impediments.
However, statistical tests revealed no difference in insurance coverage between the Exposed and Comparison groups in the US, invalidating it as an explanatory factor for the differing rates of adequate care between the cohorts. In fact, the vast majority of the US cohort had health insurance, regardless of MA-exposure status, with only 6 (1.6 %) of the total 378 participants lacking coverage. Therefore, insurance coverage does not seem to explain the disparity in adequate care.
Our findings confirm previous conclusions: surveys examining perceived barriers to prenatal care among low-income women found that financial factors, including insurance coverage, were not identified as major barriers [29, 58]. These and other detailed examinations of Med-icaid and other government health plans [59–64] in the US have found that insurance coverage does not in and of itself ensure early, regular, or adequate prenatal care, and that other environmental factors play a substantial role in determining access to care [49, 65]. Similarly, we conclude that the disparities in adequate prenatal care between NZ and the US cannot be wholly explained by a gross structural difference in the healthcare systems.
Other commonly associated maternal sociodemographic factors such as racial/ethnic minority status [11, 36, 38, 39, 48, 66–68], maternal age [11, 48, 59, 68], education level [39, 46, 48, 50, 59, 66, 68], socioeconomic status [34, 39, 42, 45, 59, 66–68], partner status [35, 42, 45, 48, 59, 68] and referral to CPS were examined along with MA-exposure and other prenatal substance use as factors related to inadequate prenatal care in both countries. Interestingly, none of these variables were significantly associated with inadequate prenatal care in either country except for CPS referral in the US, with referral increasing the odds of inadequate prenatal care 7.15 times.
Previous studies have suggested that the more interventionist mandatory reporting practices in the US [69, 70]—reflected here in increased frequency of ‘Drug Only’ CPS referrals and the significantly higher rates of subsequent out-of-home placement in the US—act as a severe disincentive for substance-using mothers seeking prenatal care. Qualitative studies have emphasized that drug-using women actively avoid or delay prenatal care out of fear of report and legal reprisal [21–29]. Medical [21, 71, 72] and legal professionals [73–76] alike have argued that these punitive legislative policies promote a ‘flight from care’ of vulnerable drug-using mothers.
However, because our study did not enquire about maternal attitudes towards CPS or reasons for delayed and/ or missed prenatal appointments, and especially because our current measure of CPS referral occurs after the period of prenatal care, it is impossible to directly test whether a fear of CPS referral prevented mothers in the US from seeking prenatal care.
Furthermore, our findings do not wholly negate the role of maternal sociodemographic factors in the receipt of inadequate prenatal care in the general population. Our focus is primarily on exploring inadequate care in the specific context of MA-exposure: namely, explaining why a disparity in adequate care among MA-using and non-using mothers exists in the US but not in NZ. Consequently, our results indicate that in this context and in our study population, other variables, such as MA-exposure and CPS referral, eclipse maternal sociodemographics in explanatory power for inadequate prenatal care. Without these factors, we may see the same demographic-dependent disparities in prenatal care reported in previous literature. Additionally, due to our recruitment methods, the mothers in the US Exposed cohort may not be nationally representative of MA-using mothers, especially regarding insurance coverage. Because the study specifically matched the Exposed and Comparison groups by insurance type, it is possible that the reported equity in coverage does not exist in the general population.
These limitations notwithstanding, our findings should encourage further research to investigate whether the US policy of mandatory report provokes a ‘flight from care’ among drug-using mothers and unintentionally exacerbates disparities in prenatal care use. Furthermore, strategies should be developed to increase access to and utilization of proper prenatal care for all women, especially those who use substances during pregnancy. Prenatal care should be seen as a potential intervention opportunity to reduce drug use.
Acknowledgments
The official name of the project is Prenatal Methamphetamine Exposure and Child Development in New Zealand and USA. This study was supported by a grant from the National Institute on Drug Abuse (Grant #R01DA021757) and a US Graduate Student grant from the Fulbright New Zealand Programme. We thank Carolyn Ho, Jenny Rogers, Jo Cliffe, Sue Cumming, Gillian Gee, Christine Todd, and Heather Stewart in Auckland, New Zealand for their assistance in this international collaboration.
Contributor Information
Min Wu, Brown Center for the Study of Children at Risk, Warren Alpert Medical School at Brown University and Women and Infants Hospital, Providence, RI, USA, min_wu@hms.harvard.edu.
Linda L. LaGasse, Brown Center for the Study of Children at Risk, Warren Alpert Medical School at Brown University and Women and Infants Hospital, Providence, RI, USA
Trecia A. Wouldes, Department of Psychological Medicine, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
Amelia M. Arria, Family Science Department, Center on Young Adult Health and Development, University of Maryland School of Public Health, College Park, MD, USA
Tara Wilcox, Brown Center for the Study of Children at Risk, Warren Alpert Medical School at Brown University and Women and Infants Hospital, Providence, RI, USA.
Chris Derauf, Department of Pediatrics, John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, USA.
Elana Newman, Department of Psychology, The University of Tulsa, Tulsa, OK, USA.
Rizwan Shah, Blank Hospital Regional Child Protection Center - Iowa Health, Des Moines, IA, USA.
Lynne M. Smith, Department of Pediatrics, LABioMed Institute at Harbor-UCLA Medical Center and David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
Charles R. Neal, Department of Pediatrics, John A. Burns School of Medicine, University of Hawaii, Honolulu, HI, USA
Marilyn A. Huestis, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, USA
Sheri DellaGrotta, Brown Center for the Study of Children at Risk, Warren Alpert Medical School at Brown University and Women and Infants Hospital, Providence, RI, USA.
Barry M. Lester, Brown Center for the Study of Children at Risk, Warren Alpert Medical School at Brown University and Women and Infants Hospital, Providence, RI, USA
References
- 1.Lagasse LL, Wouldes T, Newman E, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology and Teratology. 2010;33(1):166–175. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales R, Mooney L, Rawson RA. The meth-amphetamine problem in the United States. Annual Review of Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe-Galloway S, Ryan S, Hansen K, et al. Effects of methamphetamine abuse beyond individual users. Journal of Psychoactive Drugs. 2009;41(3):241–248. doi: 10.1080/02791072.2009.10400534. [DOI] [PubMed] [Google Scholar]
- 4.McKetin R, Kozel N, Douglas J, et al. The rise of methamphetamine in Southeast and East Asia. Drug and Alcohol Review. 2008;27(3):220–228. doi: 10.1080/09595230801923710. [DOI] [PubMed] [Google Scholar]
- 5.Crime UNOoDa. World Drug Report 2007. New York: United Nations Publications 2007; 2007. [Google Scholar]
- 6.Terplan M, Smith EJ, Kozloski MJ, et al. Meth-amphetamine use among pregnant women. Obstetrics and Gynecology. 2009;113(6):1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 7.Arria AM, Derauf C, Lagasse LL, et al. Metham-phetamine and other substance use during pregnancy: Preliminary estimates from the Infant Development, Environment, and Lifestyle (Ideal) Study. Maternal and Child Health Journal. 2006;10(3):293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 8.Wilkins C, Bhatta K, Casswell S. The emergence of amphetamine use in New Zealand: Findings from the 1998 and 2001 national drug surveys. The New Zealand Medical Journal. 2002;115(1166):U256. [PubMed] [Google Scholar]
- 9.Wilkins C, Sweetsur P. Trends in population drug use in New Zealand: Findings from National Household Surveying of Drug Use in 1998, 2001, 2003, and 2006. The New Zealand Medical Journal. 2008;121(1274):61–71. [PubMed] [Google Scholar]
- 10.Wouldes T, LaGasse L, Sheridan J, et al. Maternal methamphetamine use during pregnancy and child outcome: What do we know? The New Zealand Medical Journal. 2004;117(1206):U1180. [PubMed] [Google Scholar]
- 11.Brady TM, Visscher W, Feder M, et al. Maternal drug use and the timing of prenatal care. Journal of Health Care for the Poor and Underserved. 2003;14(4):588–607. doi: 10.1353/hpu.2010.0700. [DOI] [PubMed] [Google Scholar]
- 12.Shieh C, Kravitz M. Severity of drug use, initiation of prenatal care, and maternal-fetal attachment in pregnant marijuana and cocaine/heroin users. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2006;35(4):499–508. doi: 10.1111/j.1552-6909.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 13.Minnes S, Singer LT, Humphrey-Wall R, et al. Psychosocial and behavioral factors related to the post-partum placements of infants born to cocaine-using women. Child Abuse and Neglect. 2008;32(3):353–366. doi: 10.1016/j.chiabu.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenson AB, Wilkinson GS, Lopez LA. Effects of prenatal care on neonates born to drug-using women. Substance Use and Misuse. 1996;31(8):1063–1076. doi: 10.3109/10826089609072288. [DOI] [PubMed] [Google Scholar]
- 15.Burkett G, Gomez-Marin O, Yasin SY, et al. Prenatal care in cocaine-exposed pregnancies. Obstetrics and Gynecology. 1998;92(2):193–200. doi: 10.1016/s0029-7844(98)00202-6. [DOI] [PubMed] [Google Scholar]
- 16.El-Mohandes A, Herman AA, Nabil El-Khorazaty M, et al. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. Journal of Perinatology. 2003;23(5):354–360. doi: 10.1038/sj.jp.7210933. [DOI] [PubMed] [Google Scholar]
- 17.MacGregor SN, Keith LG, Bachicha JA, et al. Cocaine abuse during pregnancy: Correlation between prenatal care and perinatal outcome. Obstetrics and Gynecology. 1989;74(6):882–885. [PubMed] [Google Scholar]
- 18.Quinlivan JA, Evans SF. The impact of continuing illegal drug use on teenage pregnancy outcomes-a prospective cohort study. British Journal of Obstetrics and Gynaecology. 2002;109(10):1148–1153. doi: 10.1111/j.1471-0528.2002.01536.x. [DOI] [PubMed] [Google Scholar]
- 19.Racine A, Joyce T, Anderson R. The association between prenatal care and birth weight among women exposed to cocaine in New York City. Journal of the American Medical Association. 1993;270(13):1581–1586. [PubMed] [Google Scholar]
- 20.Chazotte C, Youchah J, Freda MC. Cocaine using during pregnancy and low birth weight: The impact of prenatal care and drug treatment. Seminars in Perinatology. 1995;19(4):293–300. doi: 10.1016/s0146-0005(05)80044-8. [DOI] [PubMed] [Google Scholar]
- 21.Poland ML, Dombrowski MP, Ager JW, et al. Punishing pregnant drug users: enhancing the flight from care. Drug and Alcohol Dependence. 1993;31(3):199–203. doi: 10.1016/0376-8716(93)90001-7. [DOI] [PubMed] [Google Scholar]
- 22.Downe S, Finlayson K, Walsh D, et al. ‘Weighing up and balancing out’: A meta-synthesis of barriers to antenatal care for marginalised women in high-income countries. British Journal of Obstetrics and Gynaecology. 2009;116(4):518–529. doi: 10.1111/j.1471-0528.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 23.Grandy MCT, Duerden J, Mannion K. Northern and Yorkshire Public Health Observatory WRI, University of Durham Queen’s Campus, University of Boulevard, Stockton on Tees editor. 2002. Drug misuse in pregnancy in the Northern and Yorkshire region. [Google Scholar]
- 24.Milligan R, Wingrove BK, Richards L, et al. Perceptions about prenatal care: Views of urban vulnerable groups. BMC Public Health. 2002;2:25. doi: 10.1186/1471-2458-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reis J, Mills-Thomas B, Robinson D, et al. An inner-city community’s perspective on infant mortality and prenatal care. Public Health Nursing. 1992;9(4):248–256. doi: 10.1111/j.1525-1446.1992.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts S, Pies C. Complex calculations: How drug use during pregnancy becomes a barrier to prenatal care. Maternal and Child Health Journal. 2011;15(3):333–341. doi: 10.1007/s10995-010-0594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts SCM. Re-contextualizing universal screening for alcohol and drug use in prenatal care: The (not so) hidden connections between universal screening and reporting to child protective services. Berkeley, CA: University of California, Berkeley; 2009. [Google Scholar]
- 28.Roberts SCM, Nuru-Jeter A. Women’s perspectives on screening for alcohol and drug use in prenatal care. Women’s Health Issues: Official Publication of the Jacobs Institute of Women’s Health. 2010;20(3):193–200. doi: 10.1016/j.whi.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schempf AH, Strobino DM. Drug use and limited prenatal care: An examination of responsible barriers. American Journal of Obstetrics and Gynecology. 2009;200(4):412.e1–412.e10. doi: 10.1016/j.ajog.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 30.Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: Time for policy to catch up with research. Harm Reduction Journal. 2004;1(1):5. doi: 10.1186/1477-7517-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Della Grotta S, LaGasse L, Arria A, et al. Patterns of methamphetamine use during pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Maternal and Child Health Journal. 2010;14(4):519–527. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine, National Academy of Sciences. Infant deaths, an analysis by maternal risk and health care. In: Contrasts in health status Vol. I, 1973. Chicago: Based on: The American College of Obstetricians and Gynecologists: Standards for Obstetric-Gynecologic Services; 1974. [Google Scholar]
- 33.Johnson AA, El-Khorazaty MN, Hatcher BJ, et al. Determinants of late prenatal care initiation by African American women in Washington. Dc. Maternal and Child Health Journal. 2003;7(2):103–114. doi: 10.1023/a:1023816927045. [DOI] [PubMed] [Google Scholar]
- 34.Funkhouser AW, Butz AM, Feng TI, et al. Prenatal care and drug use in pregnant women. Drug and Alcohol Dependence. 1993;33(1):1–9. doi: 10.1016/0376-8716(93)90027-n. [DOI] [PubMed] [Google Scholar]
- 35.Melnikow J, Alemagno SA, Rottman C, et al. Characteristics of inner-city women giving birth with little or no prenatal care: A case-control study. Journal of Family Practice. 1991;32(3):283–286. [PubMed] [Google Scholar]
- 36.Kalmuss D, Fennelly K. Barriers to prenatal care among low-income women in New York City. Family Planning Perspectives. 1990;22(5):215–218. 231. [PubMed] [Google Scholar]
- 37.Maupin JR, Lyman R, Fatsis J, et al. Characteristics of women who deliver with no prenatal care. Journal of Maternal-Fetal and Neonatal Medicine. 2004;16(1):45–50. doi: 10.1080/14767050412331283913. [DOI] [PubMed] [Google Scholar]
- 38.Pagnini DL, Reichman NE. Psychosocial factors and the timing of prenatal care among women in New Jersey’s healthstart program. Family Planning Perspectives. 2000;32(2):56–64. [PubMed] [Google Scholar]
- 39.Melnikow J, Alemagno S. Adequacy of prenatal care among inner-city women. Journal of Family Practice. 1993;37(6):575–578. [PubMed] [Google Scholar]
- 40.Funai EF, White J, Lee MJ, et al. Compliance with prenatal care visits in substance abusers. Journal of Maternal-Fetal and Neonatal Medicine. 2003;14:329–332. doi: 10.1080/jmf.14.5.329.332. [DOI] [PubMed] [Google Scholar]
- 41.Shankaran S, Bauer CR, Bada HS, et al. Healthcare utilization among mothers and infants following cocaine exposure. Journal of Perinatology. 2003;23(5):361–367. doi: 10.1038/sj.jp.7210946. [DOI] [PubMed] [Google Scholar]
- 42.Scupholme A, Robertson EG, Kamons AS. Barriers to prenatal care in a multiethnic, urban sample. Journal of Nurse-Midwifery. 1991;36(2):111–116. doi: 10.1016/0091-2182(91)90060-3. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman C, Paradise J. Health insurance and access to health care in the United States. Annals of the New York Academy of Sciences. 2008;1136(1):149–160. doi: 10.1196/annals.1425.007. [DOI] [PubMed] [Google Scholar]
- 44.Marquis MS, Long SH. The role of public insurance and the public delivery system in improving birth outcomes for low-income pregnant women. Medical Care. 2002;40(11):1048–1059. doi: 10.1097/00005650-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Nothnagle M, Marchi K, Egerter S, et al. Risk factors for late or no prenatal care following medicaid expansions in California. Maternal and Child Health Journal. 2000;4(4):251–259. doi: 10.1023/a:1026647722295. [DOI] [PubMed] [Google Scholar]
- 46.Cooney JP. What determines the start of prenatal care? Prenatal care, insurance, and education. Medical Care. 1985;23(8):986–997. doi: 10.1097/00005650-198508000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Oberg CN, Lia-Hoagberg B, Hodkinson E, et al. Prenatal care comparisons among privately insured, uninsured, and medicaid-enrolled women. Public Health Reports. 1990;105(5):533–535. [PMC free article] [PubMed] [Google Scholar]
- 48.Brown S, editor. Prenatal care: Reaching mothers, reaching infants. Washington, DC: Institute of Medicine/National Academy Press; 1988. [PubMed] [Google Scholar]
- 49.Hadley J. Sicker and poorer: The consequences of being uninsured: A review of the research on the relationship between health insurance, medical care use, health, work, and income. Medical Care Research and Review. 2003;60(2 suppl):3S–75S. doi: 10.1177/1077558703254101. [DOI] [PubMed] [Google Scholar]
- 50.Joseph CL. Identification of factors associated with delayed antenatal care. Journal of the National Medical Association. 1989;81(1):57–63. [PMC free article] [PubMed] [Google Scholar]
- 51.McQuide PA, Delvaux T, Buekens P, et al. Prenatal care incentives in Europe. Journal of Public Health Policy. 1998;19(3):331–349. [PubMed] [Google Scholar]
- 52.Bueche MN. Maternal-infant health care: A comparison between the United States and West Germany. Nursing Forum. 1990;25(4):26–30. doi: 10.1111/j.1744-6198.1990.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 53.Buekens P, Kotelchuck M, Blondel B, et al. A comparison of prenatal care use in the United States and Europe. American Journal of Public Health. 1993;83(1):31–36. doi: 10.2105/ajph.83.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams BC. Social approaches to lowering infant mortality: Lessons from the European experience. Journal of Public Health Policy. 1994;15(1):18–25. [PubMed] [Google Scholar]
- 55.Miller CA. Matemal health and infant survival. Washington DC: National Center for Clinical Infant Programs; 1987. [Google Scholar]
- 56.Beeckman K, Louckx F, Putman K. Predisposing, enabling and pregnancy-related determinants of late initiation of prenatal care. Maternal and Child Health Journal. 2011;15(7):1067–1075. doi: 10.1007/s10995-010-0652-1. [DOI] [PubMed] [Google Scholar]
- 57.Blondel B, Marshall B. Poor antenatal care in 20 French districts: Risk factors and pregnancy outcome. Journal of Epidemiology and Community Health. 1998;52(8):501–506. doi: 10.1136/jech.52.8.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lia-Hoagberg B, Rode P, Skovholt CJ, et al. Barriers and motivators to prenatal care among low-income women. Social Science and Medicine. 1990;30(4):487–494. doi: 10.1016/0277-9536(90)90351-r. [DOI] [PubMed] [Google Scholar]
- 59.McDonald TP, Coburnm AF. Predictors of prenatal care utilization. Social Science and Medicine (1982) 1988;27(2):167–172. doi: 10.1016/0277-9536(88)90325-5. [DOI] [PubMed] [Google Scholar]
- 60.St Clair PA, Smeriglio VL, Alexander CS, et al. Situational and financial barriers to prenatal care in a sample of low-income, inner-city women. Public Health Reports. 1990;105(3):264–267. [PMC free article] [PubMed] [Google Scholar]
- 61.Braveman P, Bennett T, Lewis C, et al. Access to prenatal care following major medicaid eligibility expansions. Journal of the American Medical Association. 1993;269(10):1285–1289. [PubMed] [Google Scholar]
- 62.Haas JS, Udvarhelyi IS, Morris CN, et al. The effect of providing health coverage to poor uninsured pregnant women in Massachusetts. Journal of the American Medical Association. 1993;269(1):87–91. [PubMed] [Google Scholar]
- 63.Piper JM, Ray WA, Griffin MR. Effects of medicaid eligibility expansion on prenatal care and pregnancy outcome in Tennessee. Journal of the American Medical Association. 1990;264(17):2219–2223. [PubMed] [Google Scholar]
- 64.Parchment W, Weiss G, Passannante MR. Is the lack of health insurance the major barrier to early prenatal care at an inner-city hospital? Women’s Health Issues: Official Publication of the Jacobs Institute of Women’s Health. 1996;6(2):97–105. doi: 10.1016/1049-3867(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 65.Braveman P, Marchi K, Egerter S, et al. Barriers to timely prenatal care among women with insurance: The importance of prepregnancy factors. Obstetrics and Gynecology. 2000;95(6, Part 1):874–880. doi: 10.1016/s0029-7844(00)00780-8. [DOI] [PubMed] [Google Scholar]
- 66.Braveman PA, Egerter SA, Cubbin C, et al. An approach to studying social disparities in health and health care. American Journal of Public Health. 2004;94(12):2139–2148. doi: 10.2105/ajph.94.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frisbie WP, Echevarria S, Hummer RA. Prenatal care utilization among non-Hispanic Whites, African Americans, and Mexican Americans. Maternal and Child Health Journal. 2001;5(1):21–33. doi: 10.1023/a:1011393717603. [DOI] [PubMed] [Google Scholar]
- 68.Goldenberg RL, Patterson ET, Freese MP. Maternal demographic, situational and psychosocial factors and their relationship to enrollment in prenatal care: A review of the literature. Women and Health. 1992;19(2–3):133–151. doi: 10.1300/J013v19n02_08. [DOI] [PubMed] [Google Scholar]
- 69.Mathews B, Kenny MC. Mandatory reporting legislation in the United States, Canada, and Australia: A cross-jurisdictional review of key features, differences, and issues. Child Maltreatment. 2008;13(1):50–63. doi: 10.1177/1077559507310613. [DOI] [PubMed] [Google Scholar]
- 70.Gateway CWI. Definitions of child abuse and neglect: Summary of state laws. Washington, DC: U.S. Department of Health and Human Services Administration for Children and Families, Children’s Bureau; 2009. [Google Scholar]
- 71.Lester BM, Twomey JE. Treatment of substance abuse during pregnancy. Women’s Health. 2007;4(1):67–77. doi: 10.2217/17455057.4.1.67. [DOI] [PubMed] [Google Scholar]
- 72.Campbell DE, Fleischman AR. Ethical challenges in medical care for the pregnant substance abuser. Clinical Obstetrics and Gynecology. 1992;35(4):803–812. doi: 10.1097/00003081-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Rubenstein L. Prosecuting maternal substance abusers: An unjustified and ineffective policy. Yale Law and Policy Review. 1991;9(1):130–160. [PubMed] [Google Scholar]
- 74.Coleman E, Miller MK. Assessing legal responses to prenatal drug use: Can therapeutic responses produce more positive outcomes than punitive responses. Journal of Law and Health. 2006;20(35):35–67. [Google Scholar]
- 75.Barth RP. Research outcomes of prenatal substance exposure and the need to review policies and procedures regarding child abuse reporting. Child Welfare. 2001;80(2):275–296. [PubMed] [Google Scholar]
- 76.Madden RG. State actions to control fetal abuse: Ramifications for child welfare practice. Child Welfare. 1993;72(2):129–140. [PubMed] [Google Scholar]