Figure 9.
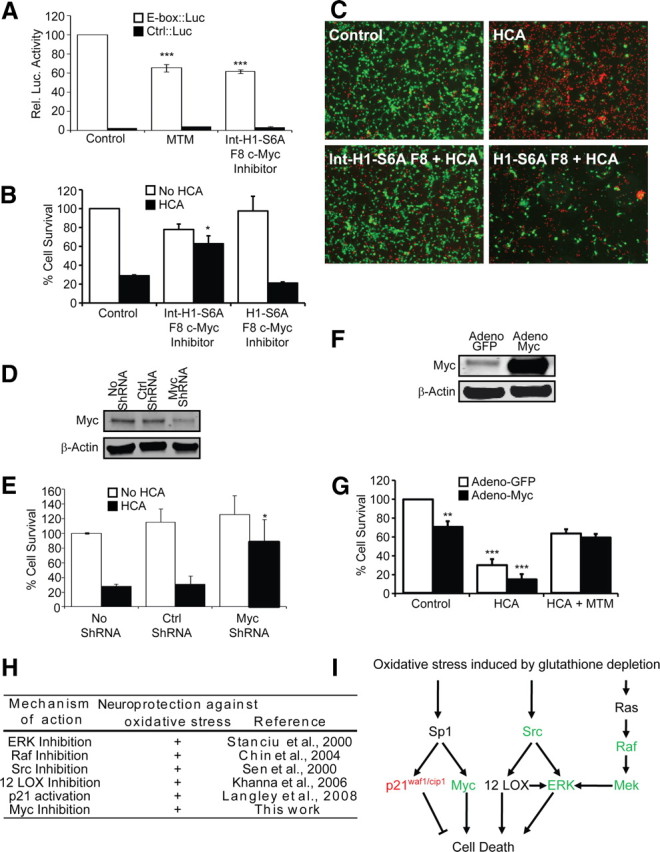
Myc suppression is sufficient but not necessary to mediate the protective effects of MTM in immature cortical neurons exposed to oxidative stress. A, Protective doses of both MTM (300 nm) and Int–H1–S6A F8 c-myc inhibitor (40 μm) significantly inhibit the expression of an E-box driving luciferase construct (Clontech). ***p < 0.0001. B, Treatment with Int–H1–S6A F8 c-myc inhibitor (40 μm) significantly protects cells from oxidative stress-induced death. The negative control peptide (H1–S6A F8 c-myc inhibitor, 40 μm) has no effect. *p < 0.05. C, Live/Dead staining of treated neurons. D, Western blots showing a significant reduction in Myc protein levels in cells expressing Myc shRNA compared with control shRNA. E, Inhibition of Myc expression using shRNA protects immature primary cortical neurons (E17) from oxidative stress-induced death. *p < 0.05. F, Western blots showing that Myc protein is increased in immature primary cortical neurons (E17) infected with the Myc2 adenovirus. G, Overexpression of Myc2 in immature primary cortical neurons (E17) significantly affects their survival but is not sufficient to block the ability of MTM to protect them from oxidative stress. Statistical analysis was conducted by one-way ANOVA followed by the Dunnett's post hoc test. Significant protection compared with control cells infected with Adeno-GFP: **p < 0.01 and ***p < 0.0001. H, Table highlighting the multiple signaling molecules targeted by protective doses of MTM and its analogs and shown to modulate neuroprotection in our model of oxidative stress. I, Model depicting pathways affecting oxidative death in neurons and that are targeted by MTM, SDK, and SK. Expression of genes depicted in green is reduced, whereas expression of genes depicted in red is induced by protective doses of MTM.
