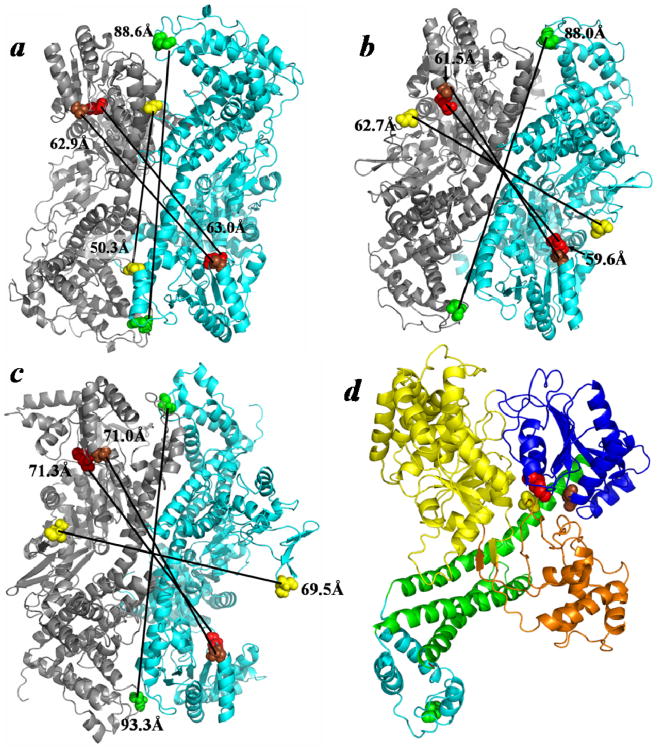
Figure 7.
Comparison of the ‘closed’ and ‘open’ states of the SecA antiparallel dimer. (A) The ‘closed’ B. subtilis SecA anti-parallel dimer (PDB ID: 1M6N) is compared to ‘open’ dimers that were modeled utilizing this preferred interface and either the (B) ‘open’ B. subtilis SecA monomer (PDB ID: 1TF5) or (C) ‘open’ E. coli SecA monomer (PDB ID: 2VDA). (D) The SecYEG-bound SecA monomer (PDB ID: 3DIN) colored according to domain: NBF1-yellow, NBF2-dark blue, PPXD-orange, HSD-green, and HWD-cyan. The color coordination for the residues, in E. coli SecA coordinates, is as follows: 340-yellow, 458-brown, 506-red, and 696-green. Predicted distances between identical residues of adjacent protomers are indicated.