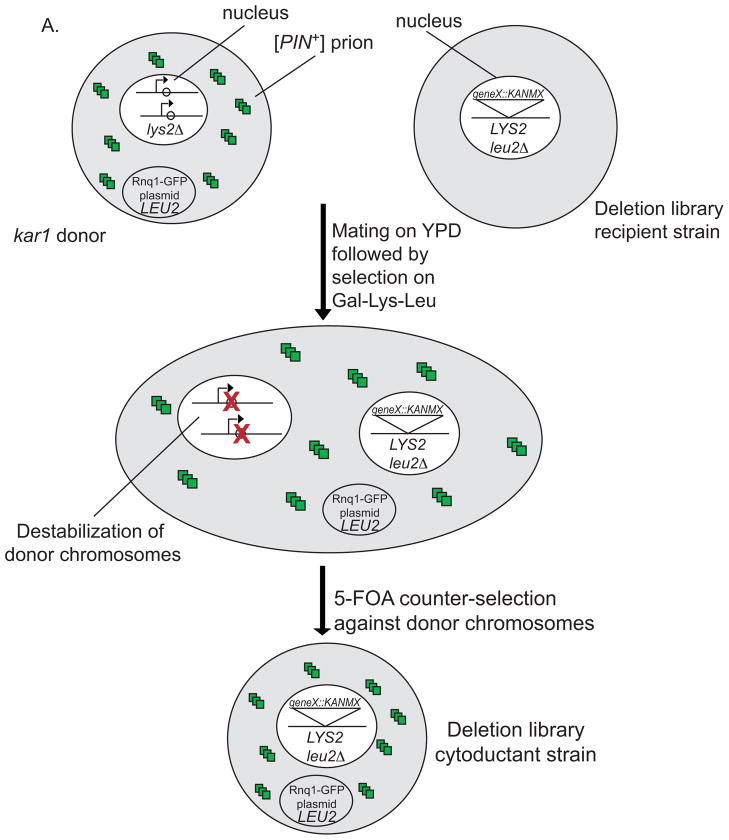
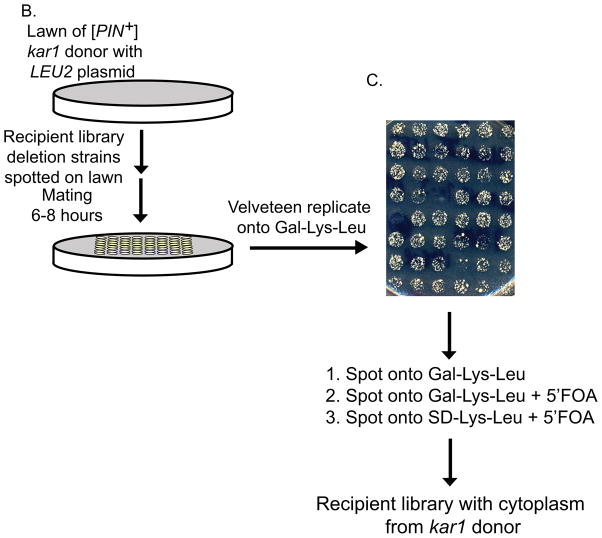
Figure 1. Cytoduction using the kar1 plasmid donor.
A. Each chromosome of the kar1 plasmid donor (top left) contains a wild type K. lactis URA3 gene and a neighboring GAL1 promoter that has been cloned adjacent to the centromere (arrow, Reid et al., 2008). The [PIN+] (small squares) lys2Δ kar1 plasmid donor that contains the LEU2 RNQ1:GFP plasmid forms heterokaryons (center) when mated to the LYS2 leu2Δ deletion library recipient strain (top right). While the cytoplasmic contents of donor and recipient cells mix, nuclear fusion is inhibited by the presence of the kar1Δ15 allele in the donor nucleus (see materials and methods). Upon plating on Gal-Lys-Leu, transcription from the GAL1 promoter through the centromeres destabilizes the chromosomes from the donor nucleus while −Lys selects against the donor cells and −Leu selects for the plasmid originally in the donor cell. Successive growth on media containing 5′ FOA (see part C) selects against any remaining chromosomes from the donor. The resulting cytoductants contain the Lys+ recipient nucleus with no donor cell chromosomes and the LEU2 RNQ1:GFP plasmid from the donor cell (bottom). B) A thin lawn of the kar1 plasmid donor was grown overnight, and the candidate strains were directly pinned onto the lawn and mated for six to eight hours. C). Mated cells were velveteen replicated onto Gal-Leu-Lys media and allowed to grow for five to seven days. While pilot studies have shown that the majority of cells within the spot are cytoductants, small amounts of donors and diploids were detected (data not shown). To eliminate remaining donors and diploids, cells were spotted again on Gal-Leu-Lys, and then spotted onto Gal-Leu-Lys+FOA followed by SD-Leu-Lys+FOA to remove any cytoductants carrying donor chromosomes from the population.