Abstract
Cannabis is the most widely used illicit drug throughout the developed world and there is consistent evidence of heritable influences on multiple stages of cannabis involvement including initiation of use and abuse/dependence. In this paper, we describe the methodology and preliminary results of a large-scale interview study of 3,824 young adult twins (born 1972–1979) and their siblings. Cannabis use was common with 75.2% of males and 64.7% of females reporting some lifetime use of cannabis while 24.5% of males and 11.8% of females reported meeting criteria for DSM-IV cannabis abuse or dependence. Rates of other drug use disorders and common psychiatric conditions were highly correlated with extent of cannabis involvement and there was consistent evidence of heritable influences across a range of cannabis phenotypes including early (≤15 years) opportunity to use (h2 = 72%), early (≤16 years) onset use (h2 = 80%), using cannabis 11+ times lifetime (h2 = 76%), and DSM abuse/dependence (h2 = 72%). Early age of onset of cannabis use was strongly associated with increased rates of subsequent use of other illicit drugs and with illicit drug abuse/dependence; further analyses indicating that some component of this association may have been mediated by increasing exposure to and opportunity to use other illicit drugs.
Keywords: Cannabis, twin, Comorbidity, Illicit drugs
Cannabis is the most widely used illicit drug in most developed countries (Degenhardt et al., 2011; Dennis et al., 2002; Hall & Degenhardt, 2007). Current (2010) US estimates suggest that 43.8% of 12th grade students have ever used cannabis, 34.8% have used it in the last year, and 21.4% have used it in the past month (Johnston et al., 2011). Slightly higher figures have been reported for Australia: 56.6% of 17-year-old males and 54.2% of 17-year-old females reported lifetime use of cannabis while 6.8% of male and 3.1% of female 12–17 year olds reported using the drug on at least 40 occasions in the past year (Lynskey et al., 1999). While many people who have used cannabis do so only infrequently, a substantial minority use cannabis heavily and experience a range of adverse consequences as a result and the potential of cannabis to be associated with abuse and dependence is recognized in both the Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association, 1994) and the International Classification of Diseases (World Health Organization, 2007). The estimated prevalence of cannabis dependence has varied widely with lifetime prevalence estimated in the region of 0.9–2.2% of the adult population (Kendler & Prescott, 1998; Perkonigg et al., 1999) while, seemingly paradoxically, the 12-month prevalence of cannabis dependence has been estimated to be as high as 9% (Chen et al., 1997; Coffey et al., 2002; Fergusson & Horwood, 2000; Teesson et al., 2002).
Heritability of Cannabis Use and Cannabis Use Disorders
In recent years there has been a growing accumulation of studies examining the heritability of cannabis-related phenotypes. Verweij et al. (2010) identified 28 studies reporting the heritability of cannabis use initiation and 27 studies reporting the heritability of problematic cannabis use (typically abuse or dependence symptomatology). Summing results from these studies using meta-analytic techniques, they reported that for initiation of cannabis use, 48% of variance in liability in males and 40% of the variance in females could be attributed to additive genetic factors while the estimates for problematic cannabis use were 51% and 59%, respectively. Modest genetic influences on availability of cannabis have also been reported (Gillespie et al., 2007).
Comorbidity
Cannabis use and cannabis use disorders are frequently comorbid with other aspects of substance use and psychiatric disorders (Kandel et al., 2001). Limited genetically informative research has suggested that a substantial co-morbidity can be explained by common or shared genetic factors. For example, conduct disorder and other externalizing problems often co-aggregate with early cannabis use and cannabis use disorders (Deas & Brown, 2006; Meyers & Dick, 2010). Additionally, Shelton and colleagues (Shelton et al., 2007) found that genes influencing conduct problems during childhood were modestly but significantly correlated with subsequent liability to cannabis use and misuse. Similarly, Lynskey et al. (2004) reported a significant genetic correlation between cannabis use disorders and major depressive disorder (MDD), and suggested that previously documented associations between early onset cannabis use and MDD could be largely attributed to the influence of shared genetic risk factors.
Gateway Effects
One of the most enduring controversies in drug research and policy concerns whether early cannabis use predisposes to the use of other drugs such as cocaine and heroin (Hall & Lynskey, 2005; Kandel, 2002; Kandel & Faust, 1975; Morral et al., 2002). Several researchers have reported strong associations between early cannabis use and subsequent use of other drugs, even after control for a wide range of potentially confounding covariates (Fergusson & Horwood, 2000; Fergusson et al., 2002; Kandel et al., 1992). However, these analyses may not have adequately controlled for important family background factors, including potential genetic in-fluences, predisposing to both early cannabis use and later use of other illicit drugs. In an earlier study of this issue employing a discordant twin design we reported, contrary to expectations that, relative to their nonearly using co-twins, individuals who commenced cannabis use prior to age 17 had significantly elevated rates of illicit drug use and illicit drug dependence, suggesting that the observed associations between early onset cannabis use and later illicit drug use cannot be solely attributed to genetic and shared environmental liabilities (Lynskey et al., 2003). Further, in a replication of this study employing sample of Dutch twins discordant for early onset use, we again found elevated rates of subsequent illicit drug use in individuals who reported early onset cannabis use, relative to their nonearly using co-twin (Lynskey et al., 2006) indicating that the relative tolerance toward marijuana use in the Netherlands did not modify the links between cannabis and other illicit drug use.
In 2005, we received a 5-year independent investigator grant (R01) from National Institute on Drug Abuse (DA18267), the principal aims of which were to examine the links between age of onset of cannabis use and risks for the subsequent development of other drug use and abuse/dependence. A key goal of the current study was to collect extensive data on patterns of early onset cannabis use and subsequent use and abuse/dependence on other illicit drugs to provide a rigorous test of the gateway hypothesis within a genetically informative research design.
Current Aims
Against this general background, aims of this study were to conduct a new, large-scale epidemiological survey of a national sample of Australian twins, previously never interviewed, to collect detailed phenotypic information on patterns of cannabis use and cannabis-related symptomatology, associated use, abuse, and dependence on both licit and illicit drugs; psychiatric comorbidity including conduct disorder, MDD, social phobia, and panic disorder; child and family circumstances including opportunity to use drugs, exposure to interparental conflict, parenting practices, peer affiliations, and adult role transitions. Below we describe the methodology of this study and provide a series of results detailing:
The extent of cannabis involvement in this sample.
The comorbidity between cannabis involvement and psychiatric morbidity.
Associations between age of onset of cannabis use and subsequent risks of other illicit drug use and drug abuse/dependence (including tobacco and alcohol).
The heritability of selected cannabis-related phenotypes including: early opportunity to use cannabis, early onset cannabis use, lifetime use of cannabis on 11 or more occasions, and meeting criteria for a DSM diagnosis of cannabis abuse or dependence.
Method
Sample
At the time this study began (2005), the volunteer Australian Twin Registry (ATR) had enrolled a total of 4,131 twin pairs born 1972–1979. Attempts to recruit these individuals to participate in the current cannabis study were made using two-tiered process, as required by the ATR’s ethics committee: first, the ATR contacted twins (by mail and subsequently by telephone) and asked if they were willing to have their name and contact details forwarded to the Queens-land Institute of Medical Research (QIMR) for potential participation in an interview-based study of substance use and mental health. Contact details of those consenting to such were then forwarded to QIMR who made separate attempts to (re)contact potential subjects, explain the purposes of the study to them, and enroll them in the study. Due to a lower than anticipated initial response from enrolled twins, efforts were made in the final phases of data collection to identify and recruit siblings of participating twins as there are well known increases in statistical power for genetically informative research design including three or more related individuals.
The ATR approached 7,850 twins born between 1972 and 1979, of these 3,786 twins consented to participate, 1,971 declined and the reminder gave passive refusals (5 attempts/calls without reaching respondent) or did not respond. In addition, upon interview with the twins, 1,218 siblings were eligible for participation and 849 were recruited of which 424 siblings completed both the interview and questionnaire. Siblings were recruited by QIMR staff and at least 10–15 attempts were made to contact siblings before classifying them as passive refusals. Respondents were also contacted weekly, over a period of 3 months, regarding the questionnaire. On completion of both the interview and questionnaire, all respondents received a $50 gift card. Table 1 provides the distribution of twins and siblings, by sex and paired status, who participated in the interview and questionnaire components of the project.
TABLE 1.
Distribution of Targeted Subjects and Those Completing the Interview and Questionnaire Phases of the Study
| N in study | Completed IV +Q | Completed IV (includes partials) | Completed Q | ||
|---|---|---|---|---|---|
| Sex | Males | 2,020 | 1,224 | 1,389 | 1,257 |
| Females | 2,958 | 2,330 | 2,435 | 2,368 | |
| Total Rs | 5,004 | 3,554 | 3,824 | 3,625 | |
| Twins | Male twins | 1,418 | 1,047 | 1,180 | 1,074 |
| Female twins | 2,368 | 2,083 | 2,168 | 2,112 | |
| Total twins | 3,786 | 3,130 | 3,348 | 3,186 | |
| Siblings | Male siblings | 602 | 177 | 209 | 183 |
| Female siblings | 590 | 247 | 267 | 256 | |
| Total siblings | 1,218a | 424 | 476 | 439 | |
| Pairs | Pairs | 1,525 | 1,116 | 1,233 | 1,151 |
| Both pairs | 3,050 | 2,232 | 2,466 | 2,302 | |
| One of pair only | NA | 329 | 250 | 304 | |
| Singleton | 736 | 569 | 632 | 580 | |
| Siblings | 1,218 | 424 | 476 | 439 | |
| Total Respondents | 5,004 | 3,554 | 3,824 | 3,625 | |
| Siblings with consent to be approached | 849 | ||||
Note:
Some numbers in sex breakdown do not sum to total as sex of some siblings is unknown.
The final sample comprised 3,824 participants from a target sample of 5,004 individuals who provided with consent for QIMR to approach them and included: 3,348 twin individuals, including: (a) 976 monozygotic female (MZF), with 398 pairs and 180 unpaired twins; (b) 490 monozy-gotic male (MZM), with 173 pairs and 144 unpaired twins; (c) 741 dizygotic female (DZF), with 305 pairs and 131 unpaired twins; (d) 373 dizygotic male (DZM), with 118 pairs and 137 unpaired twins; and (e) 746 dizygotic opposite sex (DZOS), with 231 pairs and 284 unpaired twins. There were 22 twins with undetermined zygosity and 476 nontwin siblings.
Assessment Protocol
The assessment protocol was based on the semi-structured assessment of the genetics of alcoholism (Australian version; SSAGA-OZ) and administered using a computer-administered telephone interview (Bucholz et al., 1994; Heath et al., 1997) used in a previous ‘1989’ cohort of Australian twins born between 1964 and 1971 (Knopik et al., 2004). This instrument obtains full diagnostic criteria for cannabis and other illicit drug abuse and dependence. In addition, we assessed specific aspects of experience with cannabis and other illicit drugs, including exposure opportunity, initial subjective reactions to cannabis and components of cannabis withdrawal, and full DSM-IV diagnostic criteria for a number of other diagnoses (MDD, childhood conduct disorder, alcohol and nicotine dependence, panic disorder). The SSAGA, from which this interview assessment was derived, has been shown to have good reliability and validity as a measure of standardized DSM-IV diagnostic criteria for a range of conditions including alcohol abuse and dependence, nicotine dependence, MDD, and antisocial personality disorder (Bucholz et al., 1994).
In addition, respondents were requested to complete a brief questionnaire which included questions on physical health, personality, and other measures. These items were assessed using a questionnaire rather than during the interview to reduce the length of the interview. Respondents were offered two methods for completing the questionnaire survey: either by completing a ‘paper and pen’ survey and returning it to QIMR via post or by completing the survey over the Internet (after logging on with a unique ID and password, supplied by QIMR). Response rates for the questionnaire were excellent: 93% of those interviewed also completed the questionnaire, with 75% of those opting to complete the Internet version of the questionnaire.
The assessment protocol is summarized in Table 2 and key features are highlighted below.
TABLE 2.
Summary of Domains Assessed
| Cannabis use |
| Age of first use opportunity |
| Age at initiation of cannabis use |
| Initial reactions to cannabis use |
| Weekly use |
| Daily use |
| Cannabis use disorders |
| Abuse (Including age at onset) |
| Dependence (DSM-IV, including withdrawal) |
| Other illicit drug use |
| Age of first use opportunity |
| Lifetime use |
| Age of onset |
| Frequency of use (when used most) |
| Abuse |
| Dependence |
| Tobacco use |
| Initiation of smoking |
| First reactions to tobacco |
| Transition to weekly smoking (age) |
| Transition to daily smoking (age) |
| Current frequency of smoking |
| Current cigarettes/day when smoked |
| Maximum cigarettes in single day |
| Tobacco dependence (DSM-IV) |
| Alcohol use |
| Initiation of alcohol use |
| First reactions to alcohol |
| Frequency of alcohol use |
| Amounts consumed (current and maximum) |
| Alcohol abuse/harmful use (DSM-IV) |
| Alcohol dependence (DSM-IV) |
| Parental alcohol use/problems |
| Proximal risk factors |
| Peer behaviors |
| Perceived peer Smoking |
| Perceived best friend’s smoking |
| Perceived peer alcohol use |
| Perceived best friend’s alcohol use |
| Perceived peer delinquent behavior |
| Perceived peer illicit drug use |
| Perceived co-workers tobacco, alcohol, and illicit drug use |
| Behavioral inhibition |
| Major depression |
| Suicidal ideation |
| Suicide attempt |
| Neuroticism (NEO) |
| Social phobia (DSM) |
| Panic disorder (DSM) |
| Agoraphobia (DSM) |
| Deliberate (nonsuicidal) self harm |
| Behavioral undercontrol |
| Childhood conduct disorder |
| Antisocial personality disorder |
| Childhood hyperactivity |
| Childhood inattention |
| Extraversion (NEO) |
| Novelty seeking (TPQ) |
| Sociodemographic |
| Household income |
| Parental educational levels |
| Parental marital status & history |
| Family structure & rearing history |
| Ethnicity |
| Religious affiliation & involvement |
| Academic achievement |
| School grades |
| Educational history/status |
| Family ‘environment’ variables |
| Perceived parental conflict |
| Perceived parent–child conflict |
| Perceived sibling smoking, alcohol, illicit drug |
| Parent–child relations — parental involvement |
| Parental coldness, overprotection |
| Parental major depression |
| Parental alcohol dependence |
| Parental discipline |
| Risk modifiers |
| Parent–child relations — parental control |
DSM-IV Cannabis Abuse
The reliability and validity of DSM cannabis abuse diagnoses derived from the SSAGA are comparable to estimates of the reliability of abuse diagnoses derived from other instruments. Thus, this unreliability likely reflects problems with the conceptualization of abuse diagnoses (Hasin & Paykin, 1999).
DSM-IV Cannabis Dependence
Cannabis dependence was assessed using SSAGA, supplemented by detailed questions, based on those described by Budney et al. (Budney et al., 2004; Budney & Hughes, 2006), to assess potential symptoms of withdrawal. A lifetime diagnosis was obtained by endorsement of 3 of 7 (including withdrawal) criteria clustering within a single 12-month period. However, as the DSM-5 operationalization of withdrawal was unknown at the time of interview, for diagnosing dependence, withdrawal was coded as endorsement of 2 or more of 7 criteria or withdrawal relief.
Other Substance use, Abuse, and Dependence
Comprehensive information on patterns of substance use and symptoms of DSM-IV abuse and dependence was assessed using the SSAGA and, for nicotine dependence, the CIDI.
(a) Lifetime Tobacco Use and Nicotine Dependence
The SSAGA-OZ includes the CIDI assessment of nicotine dependence. The test-retest reliability of the CIDI assessment of nicotine dependence has been estimated at 0.64 (World Health Organization, 1994). Items from the Heaviness of Smoking Index (Heatherton et al., 1989) were also included.
(b) Lifetime Alcohol Use, Abuse, and Dependence
The SSAGA-OZ, previously used in telephone interviews of older cohorts of Australian twins ascertained using identical procedures to those currently proposed, obtains an extensive lifetime history of the frequency of alcohol consumption and the experience of symptoms of alcohol abuse and dependence.
(c) Lifetime Use, Abuse, and Dependence on Other Illicit Drugs
DSM-IV criteria for these drug classes were also assessed using the SSAGA, which obtains full DSM-IV diagnostic criteria for abuse and dependence. SSAGA-based assessments of dependence on these substances have acceptable to good reliability and validity. Again, the reduced reliability and validity of SSAGA assessments of abuse on these substances is consistent with the estimated reliability and validity of other assessments of abuse on these substances and likely reflects limitations of the DSM operationalizations of abuse. Drug classes assessed included: (a) sedatives; (b) cocaine; (c) amphetamines and other stimulants; (d) opioids; (e) hallucinogens; (f) disassociates (phencyclidine, ketamine); (g) solvents; and (h) inhalants.
(d) Lifetime Access and Availability of Cannabis and Other Illicit Drugs
Exposure opportunity was assessed using procedures developed for earlier waves of the National Household Survey and discussed by Anthony and colleagues (Wagner & Anthony, 2002; Wilcox et al., 2002). Such questioning would precede questioning on drug use (including licit drugs) and would involve respondents reporting the age (if any) at which they first had access to each drug class (including cannabis, other illicit drugs, and the licit drugs tobacco and alcohol), irrespective of whether they used it at that occasion or not.
(e) Lifetime Psychopathology
The SSAGA was designed to collect comprehensive diagnostic information on lifetime patterns of psychiatric illness. For the current study, the SSAGA provided the basis of DSM-IV assessments of the following psychiatric disorders:
Childhood conduct disorder.
Adult antisocial personality disorder.
MDD.
Suicidal ideation and attempted suicide.
(f) Childhood and Family Environment
A range of measures of childhood and family environment were also assessed retrospectively including family history of substance use and psychiatric disorders, parent–child relationships, and parental discipline and current affiliations with delinquent or substance using peers.
(g) Zygosity
Twin pair zygosity was determined on the basis of responses to standard questionnaires about physical similarity and confusion of the twins by parents, teachers, and strangers: methods that have been found to give better than 95% agreement with results of genotyping. Information about shared early experiences (e.g., shared peers, being in the same classes at school) was also gathered to allow conservative adjustment for excess similarity of environmental experiences in monozygotic (MZ) compared to dizygotic (DZ) peers.
Use of a Computer-Assisted Telephone Interview (CATI)
A feature of the current study was that it utilized a CATI. Compared to hard paper copy interviews, CATI-based assessment has a number of advantages including: (a) minimizing interviewer errors that may lead to subject recon-tacts or missing data; (b) improved querying of respondents about their symptom histories, through visual display to the interviewer; (c) rapid editorial review to respond to interviewer queries and provide interviewer feedback; (d) rapid consultation of the project clinician to resolve diagnostic questions: (e) ease of access to interviewer comments where these may help resolve diagnostic and other issues (whereas these are recorded in hardcopy interviews, they are rarely accessed, especially in international collaborations); (f) as a consequence of (e), greater ease of making data freely available to other investigators. The average length of the interview was 98 minutes.
Quality Control Steps
The CATI program contained numerous internal edits preventing incorrect or missing entries from recorded in the interview. Internal programming also ensured that interviewers automatically ‘skipped’ to the next appropriate question. Interviewers received substantial training and feedback on their performance and attended monthly project meetings. Each interview was audiotaped (subject to participant consent) and project editors reviewed one in every 15 interviews. Feedback on these reviews was provided to interviewers.
Statistical Analyses
Descriptive statistics and chi-square tests of association were conducted in SAS (SAS Institute, 1999). Cox proportional hazards models were fitted to the data to examine the relationship between age at first cannabis use
and age at opportunity to use cocaine in STATA (Stata Corp, 2003) using stcox. The Huber–White variance estimator that adjusts standard errors for familial clustering was implemented. Univariate twin analyses were conducted in Mx (Neale, 2004) using raw ordinal data and full-information maximum-likelihood (FIML) estimation which makes use of incomplete twin pairs. Variance was attributed to additive genetic (A), shared environmental (C), and unique/nonshared environmental (E, also including measurement error) factors, with no evidence, based on MZ–DZ correlations for nonadditive genetic (D) influ-ences. Sex differences in prevalences or multiple response categories were modeled by allowing thresholds to differ between males and females. Even if these are the same between sexes, it does not necessarily follow that causes of variation in underlying liability will be the same in males and females and we also modeled this sex limitation by allowing the A, C, and E parameters to vary across sexes, including allowing for qualitative sex differences (Rg ≠ 0.5) in DZ opposite sex pairs.
Results
(1) Sample Characteristics
Demographic characteristics of the final sample (including both twins and siblings) are displayed in Table 3. More women than men participated, which is typical of twin studies. The sample was predominantly young adult with a high degree of educational attainment with only 6.5% reporting less than a high school education and 47.3% reporting some university education. Nearly 83% of the sample was employed either full- or part-time at the time of interviews.
TABLE 3.
Sociodemographic Characteristics of Full Sample (n = 3,824)
| Mean age | 32.1 [27–40 years] | |
|---|---|---|
| Female | 63.6% | |
| Married or de facto relationship | 53.8% | |
| Current religion | Catholic | 22.4 |
| Anglican | 17.2 | |
| Other Christian | 18.2 | |
| No religion | 40.7 | |
| Both parents born in Australia | 67.0 | |
| Education | 8–10 years | 6.5 |
| High school graduate | 18.0 | |
| Vocational or technical college | 28.2 | |
| University education | 27.5 | |
| Post graduate education | 19.8 | |
| Occupational status | Employed fulltime | 60.8 |
| Employed part-time | 22.1 | |
| Homemaker | 12.2 | |
| Unemployed | 1.8 | |
| Student | 2.4 | |
| Retired/disability | 0.8 | |
| Current gross personal income (AUD$ pa) | 0–24,999 | 27.4 |
| 25,000–49,999 | 27.0 | |
| 50,000–74,999 | 25.0 | |
| 75,000+ | 20.6 |
(2) The Prevalence and Extent of Cannabis Involvement
Note that, 75.2% of males and 64.7% of females reported lifetime use of cannabis. When stratifying by complete/incomplete twin pairs, cannabis use was elevated in female twins whose MZ co-twin or DZ opposite sex co-twin did not participate. While 59.8% and 64.0% of females from complete MZF and DZOS pairs reported lifetime cannabis use, respectively, 68.9% and 75.9% of singleton female twins from these zygosity groups reported lifetime cannabis use (p < .05). This pattern is expected if the variable in question is affecting compliance rate; fortunately FIML corrects for this ascertainment bias to give good estimates for the full population (Neale & Cardon, 1992; Neale et al., 1994). No other differences across zygosity groups were noted. While many individuals reporting lifetime cannabis use had used the drug relatively infrequently, prolonged, frequent, and problematic cannabis use was also relatively common in this cohort: as shown in Table 4: 23.0% of men and 11.3% of women reported using cannabis at least 100 times, 14.5% of men and 7.1% of women reported using it daily during their period of heaviest use, 23.3% of men and 10.6% of women met DSM criteria for cannabis abuse, and 14.8% (males) and 6.8% (females) met lifetime criteria for cannabis dependence. Across all these measures there were significant sex differences with some suggestion that the magnitude of these differences increased with increasing severity of cannabis involvement: for example, males were 1.66 (95% CI = 1.43–1.92) times more likely to report lifetime use but 2.44 (95% CI = 1.05–3.90) times more likely to meet criteria for cannabis abuse or dependence.
TABLE 4.
Measures of Lifetime Cannabis use (%) and Symptomatology in Males and Females (n = 3824) - Odds Ratios for Males Compared with Females are Given
| Males [n = 1,389] | Females [n = 2,435] | Odds ratio | 95% CI | |
|---|---|---|---|---|
| Opportunity to use | 93.5 | 86.2 | 2.30 | 1.81–2.94 |
| Lifetime use | 75.2 | 64.7 | 1.66 | 1.43–1.92 |
| Used 100+ times | 23.0 | 11.3 | 2.33 | 1.95–2.78 |
| Used weekly | 29.1 | 16.8 | 2.03 | 1.74–2.38 |
| Used daily | 14.5 | 7.1 | 2.23 | 1.79–2.76 |
| Cannabis abuse | 23.3 | 10.6 | 2.58 | 2.15–3.08 |
| Any DSM-IV dependence symptom | 23.7 | 12.7 | 2.12 | 1.79–2.52 |
| DSM-IV Cannabis dependence | 14.8 | 6.8 | 2.40 | 1.93–2.97 |
| Cannabis abuse/ dependence | 24.5 | 11.8 | 2.44 | 2.05–2.90 |
Of those reporting using cannabis at least 100 times, 48.5% met criteria for cannabis withdrawal as proposed by DSM-5 (i.e., experiencing 3 or more of 7 withdrawal symptoms) while 21.2% reported using withdrawal relief. Table 5 shows the prevalence of these withdrawal symptoms (considered to be endorsed if the respondent reported experiencing it some, quite a bit, or a great deal) which ranged from 47.4% for anger/aggression/irritability to 30.6% for decreased appetite. Craving during abstinence, which is not part of the proposed DSM-5 revisions, was endorsed by 53.4%. Additionally, 37.3% endorsed experiencing nausea or strange dreams. Withdrawal correlated well with other DSM-IV abuse and dependence criteria (r = 0.66, Cron-bach’s alpha = 0.81, total alpha = 0.83). In those who reported using cannabis at least 100 times, 60.4% of those meeting criteria for DSM-IV abuse or dependence also met criteria for DSM-5 proposed withdrawal.
TABLE 5.
Prevalence of Withdrawal Symptoms, as Proposed by DSM-5, in Those Reporting Use of Cannabis at Least 100 Times Across the Lifetime (n = 595)
| Withdrawal symptom | Prevalence (%) |
|---|---|
| Anger, aggression, irritability | 47.4 |
| Nervousness, anxiety | 33.4 |
| Sleep difficulties | 45.2 |
| Decreased appetite | 30.6 |
| Restlessness | 44.2 |
| Depressed mood | 41.2 |
| Physical symptoms (headaches, shakes, stomach pain, sweats) | 32.8 |
(3) Comorbidity Between Extent of Cannabis Involvement and Other Substance Use and Psychiatric Disorders
Table 6 shows the associations between the extent of cannabis use, assessed using a three-level variable distinguishing nonusers from lifetime users who either did or did not meet criteria for a diagnosis of cannabis abuse or dependence. Several features of the results displayed in this table are noteworthy: (a) there were particularly strong associations between cannabis involvement and other measures of substance involvement including dependence on the licit drugs (tobacco, alcohol) and illicit drug abuse/dependence. Similarly, there were consistent albeit less strong associations between cannabis involvement and measures of both externalizing behaviors (including a history of childhood conduct disorder, attention defict hyperactivity disorder (ADHD), and problem gambling) and internalizing behaviors (MDD, suicidal behaviors, anxiety disorders). In all comparisons those who met criteria for cannabis abuse or dependence had the highest risks of these disorders and nonusers had the lowest levels while individuals who reported having used cannabis but who did meet criteria for cannabis abuse/dependence having rates of these outcomes that were intermediate between these extremes.
TABLE 6.
Extent of Cannabis Involvement and Comorbidity with Substance Use and Other Psychiatric Disorders by Sex (n = 3,824)
| Males [n = 1,389]
|
Females [n = 2,435]
|
|||||||
|---|---|---|---|---|---|---|---|---|
| No use | Use | Abuse/ dependence | Phi* | No use | Use | Abuse/ dependence | Phi | |
| Nicotine dependence | 7.3 | 22.3 | 59.7 | 0.43 | 5.9 | 28.3 | 62.6 | 0.40 |
| Alcohol dependence | 14.6 | 35.4 | 53.8 | 0.29 | 6.2 | 23.1 | 41.6 | 0.29 |
| Other illicit drug abuse/dependence | 2.0 | 5.8 | 41.2 | 0.46 | 0.6 | 5.7 | 33.9 | 0.39 |
| Conduct disorder | 5.0 | 10.4 | 30.9 | 0.28 | 1.2 | 4.3 | 22.7 | 0.29 |
| ADHD | 7.8 | 8.4 | 14.4 | 0.09 | 5.2 | 5.9 | 15.7 | 0.13 |
| Problematic gambling | 10.6 | 13.7 | 23.2 | 0.13 | 2.1 | 4.8 | 10.8 | 0.13 |
| MDD | 10.4 | 14.6 | 27.7 | 0.18 | 23.1 | 28.1 | 48.3 | 0.17 |
| Suicidal ideation | 16.8 | 23.1 | 42.9 | 0.22 | 20.6 | 25.6 | 46.9 | 0.18 |
| Suicide attempt | 1.7 | 1.5 | 6.8 | 0.14 | 3.2 | 5.0 | 13.6 | 0.14 |
| Deliberate self-harm | 3.1 | 3.9 | 12.9 | 0.17 | 4.3 | 7.9 | 17.8 | 0.15 |
| Panic disorder | 2.2 | 2.8 | 5.0 | 0.06 NS | 4.6 | 5.7 | 12.2 | 0.10 |
| Social phobia | 1.1 | 1.7 | 3.5 | 0.06 NS | 4.5 | 4.7 | 9.4 | 0.07 |
Note:
Phi coefficient of association, where φ = √(χ2/n). For two binary measures (2 × 2 contingency table), it approximates a Pearson correlation and ranges between −1 and +1.
(4) Age of Onset of Cannabis Use and Subsequent Cannabis and Other Illicit Drug Involvement
A major focus of this project concerned the extent to which early onset cannabis use may be associated with escalation in drug use and the development of illicit drug abuse/dependence. To explore the utility of the current data for addressing these issues, we present here a series of analyses describing the age of onset of cannabis use and the links between early onset cannabis use and later risks of other illicit drug use and drug abuse/dependence. Onset of cannabis use typically occurred during the teenage years with 78% of lifetime cannabis users reporting initiating use before age 20. Again, there was evidence of very small sex differences with early onset of cannabis use being more common among males than among females: for example, 57.5% of male cannabis users reported initiating cannabis use before age 18, compared with 50.9% of females.
Figure 1 shows the associations between age of onset of cannabis use (measured as an ordinal variable) and measures of cannabis abuse/dependence, lifetime use of selected illicit drugs (cocaine, stimulants, hallucinogens), and abuse or dependence on these drug categories. This figure shows consistent — and approximately linear — associations between the age of onset of cannabis use and risks of these outcomes: individuals reporting onset of cannabis use before age 16 had rates of these outcomes that were substantially higher than rates among those initiating cannabis use after age 20. Rates of these outcomes were lowest among those reporting no lifetime use of cannabis.
FIGURE 1.
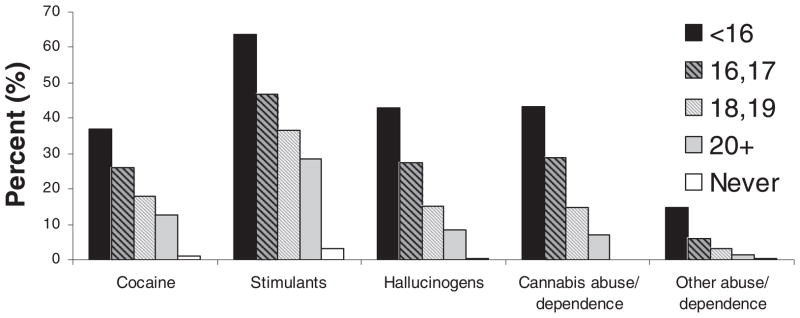
Age of onset of cannabis use and rates of illicit drug use and cannabis/other illicit drug abuse/dependence (other abuse/dependence includes cocaine, stimulants, & hallucinogens abuse/dependence) in 2,601 cannabis users.
(5) Age of Onset of Cannabis Use and Access/Opportunity to Use Other Drugs
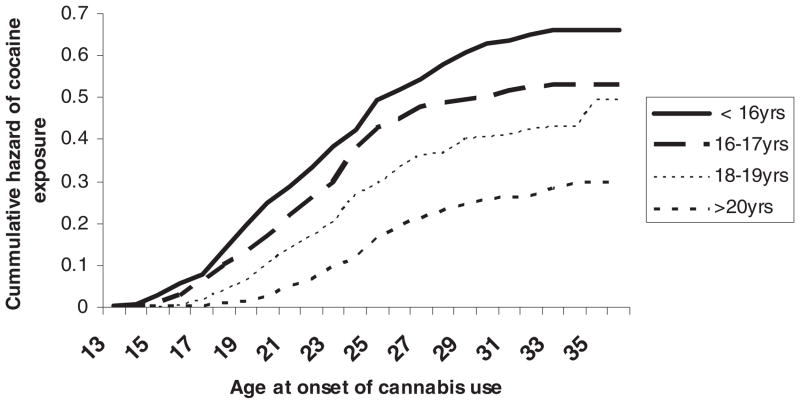
One key hypothesis described in our initial grant (and based on previous work of Anthony and colleagues; Wagner & Anthony, 2002) was that the links between early onset cannabis use and later drug use may potentially be explained by use of cannabis being associated with increased availability and opportunity to use other drugs. To examine this, we conducted a series of survival analyses in which age of onset of opportunity to use cocaine was modeled as a function of age of onset of cannabis use, divided into the five categories described above: onset before age 16, onset at ages 16, 17, onset at ages 18, 19, onset at age 20 or older, and no lifetime cannabis use. These results are summarized graphically in Figure 2 and show a strong association between the age of onset of cannabis use and the age at which subjects reported first having the opportunity to use cocaine (whether or not they did, in fact use cocaine then -– or indeed ever) (HR = 0.74, 95% CI = 0.69–0.79). Thus, the earlier an individual used cannabis for the first time, the lower the age at which they first had the opportunity to use cocaine.
FIGURE 2.
Hazards of cocaine exposure opportunity as a function of varying age at first use (onset) of cannabis use in 2,601 cannabis users.
(6) The Heritability of Cannabis-Related Phenotypes
As an initial attempt to examine the extent to which cannabis-related phenotypes are influenced by heritable factors a series of univariate genetic models were fitted to the data on: (a) early (≤15 years) opportunity to use cannabis; (b) early onset (≤16 years) use; (c) lifetime use of cannabis on 11 or more occasions, and (d) meeting DSM criteria for a lifetime diagnosis of cannabis abuse or dependence. For each of these phenotypes, the best-fitting model was one which assumed no sex differences in the magnitude of genetic or environmental effects and the results of these analyses are summarized in Table 7. It can be seen that there were substantial heritable influences on each of the cannabis-related phenotypes with the magnitude of these influences ranging from 72% (for both early onset opportunity to use and DSM abuse/dependence) to 80% (for early onset use). The best-fitting model was an AE model and there was no significant evidence for shared environmental influences on any of these measures.
TABLE 7.
Estimated Heritability of Selected Cannabis-Related Phenotypes
| A | C | E | |
|---|---|---|---|
| Early (≤15 years) opportunity to use | 72 (63–80) | 28 (20–38) | |
| Early onset (≤16 years) use | 80 (73–86) | – | 20 (14–27) |
| Used 11+ times | 76 (68–82) | – | 24 (18–32) |
| DSM abuse or dependence | 72 (59–82) | – | 28 (18–41) |
Discussion
In this paper, we have presented preliminary results from an interview study of 3,824 Australian-born twins and their nontwin siblings which included comprehensive assessments of cannabis-related phenotypes and related measures of substance use and psychiatric disorders. A striking find-ing from the current study was the high levels of cannabis involvement: some lifetime use of the drug was normative with 64.7% of females and 75.2% of males reporting ever having used the drug. These rates of use modestly exceeded rates from an independent cohort of Australian twins born between 1964 and 1971 (Lynskey et al., 2002) indicating increasing use of cannabis in Australia for cohorts born before 1990, consistent with prior findings from the Aus-tralian Household Survey (Degenhardt et al., 2000). While the majority of those reporting cannabis use had used the drug only infrequently, there were substantial minorities who reported frequent use or experiencing problems as a consequence of their cannabis use. Strikingly, 11.8% of females and 24.5% of males met criteria for DSM-IV cannabis abuse and/or dependence.
Again confirming the results of previous studies, there was a high degree of association or comorbidity between the extent of cannabis involvement and measures of other substance use and abuse/dependence and psychiatric illness. Individuals who met criteria for cannabis abuse or dependence had rates of these disorders that were 1.8–56.5 times higher than the corresponding rates among individuals who reported never using cannabis (see Table 5) while individuals who had used cannabis but did meet criteria for abuse or dependence having rates of these outcomes that were intermediate between these two groups. Multiple explanations have been advanced to explain the associations between cannabis use and other drug use/psychiatric morbidity including that cannabis use -– and particularly heavy or symptomatic use — predisposes individuals both to use other substances and to develop substance use disorders and other psychiatric disorders. Thus, for example, it has been proposed that early onset cannabis use may increase risks for the development of MDD among women (Patton et al., 2002), Alternatively, it has been suggested that drug use may develop as a result of attempts to self-medicate adverse emotional states (Khantzian, 1997). Finally, it has also been proposed that there may be no patterns of reciprocal causation between drug use and mental health but that their apparent comorbidity arises from the influence of shared and correlated risk factors that operate to increase risks of both drug use and of mental health problems. For example, our own research, based on an older cohort of Aus-tralian twins, suggests that the association between cannabis use and depression may be largely noncausal and arise from risk factors, particularly shared genetic predispositions that increase risks of both cannabis use and of developing major depression (Lynskey et al., 2004). While we have not examined mechanisms underlying the observed associations between cannabis use and other drug use/mental health in our current analyses, we hope to more fully exploit the advantages of twin data to more comprehensively address this question using the current data set.
The current analyses also demonstrated a strong inverse relationship between age of onset of cannabis use and risks of a range of drug-related outcomes including use of (other) illicit drugs and development of abuse or dependence on cannabis, other illicit drugs, and on the licit drugs tobacco and alcohol (Figure 1). For example, 36.8% of those who reported using cannabis before age 16 reported lifetime use of cocaine, compared with only 13.2% of those who commenced cannabis use after age 20 (and 1.1% of those who reported never using cannabis). Additionally, the association between age of onset of cannabis use and these outcomes was approximately linear with risks of these outcomes increasing steadily as age of onset of cannabis use declined. While such associations have commonly been reported in the literature, they remain controversial and, in particular, interpretation of these associations and of the mechanisms underlying them remains a topic of considerable debate. In addition to the advantages to studying this important question inherent in the twin design, the current study also contained several unique aspects of data collection that we hope will help elucidate these mechanisms. Preliminary analyses presented here, while not comprehensively addressing this, do suggest that more intensive analyses of the data collected here may offer valuable insights into the mechanisms underlying these associations. Specifi-cally, our data highlight the important associations between age of onset of cannabis use and increasing opportunities for the use of other illicit drugs. In future studies based on these data, we hope to explore the extent to which the well known associations between declining age of onset of cannabis use and increasing rates of other illicit drug use and drug abuse/dependence can be explained by a combination of factors including opportunity and access to other drugs as well as peer and related factors. Resolution of this issue has important implications for policy and prevention of substance-related problems.
Finally, the importance of studying such policy relevant questions within the context of genetically informative research designs was highlighted by our findings of considerable heritable influences across a range of cannabis-related phenotypes including those, such as opportunity to use cannabis, that have been traditionally perceived as environmental constructs. Nonetheless, our analyses revealed moderate heritable influences on both early age of first opportunity to use cannabis use (72%) and, importantly, on early onset cannabis use itself (80%). In parallel analyses, we also found evidence for moderate to high heritability of a measure of cannabis abuse or dependence. While there were differences between the current study and previous studies of cannabis abuse/dependence (e.g., cohort differences between samples, the inclusion of a comprehensive assessment of withdrawal within the definition of dependence), the parallels between our findings and those of previous studies are striking and there is now a convergence of evidence to suggest that between 51% and 58% of liability to develop cannabis dependence within Western societies where cannabis is commonly available can be attributed to heritable influences (Verweij et al., 2010). It therefore remains feasible that some component both of the association between age of onset of cannabis use and the subsequent use and misuse of other illicit drugs and of the comor-bidity between cannabis dependence and other psychiatric conditions (including drug dependence) may be due to a shared genetic liability between cannabis use/dependence and these other phenotypes. In subsequent studies based on these data, we hope to able to help elucidate such issues.
Acknowledgments
This research was funded by National Institute on Drug Abuse (NIDA) grant: DA18267 (ML) and facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council. We thank Dixie Statham, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, and Adele Somerville, who worked on this project and the twins and their siblings for participating.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 1994. Revised. [Google Scholar]
- Bucholz KK, Cadoret RJ, Cloninger RC, Dinwiddie SH, Hesselbrock V, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies. Journal for the Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. American Journal of Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB, Davies M. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug and Alcohol Dependence. 1997;46:53–67. doi: 10.1016/s0376-8716(97)00047-1. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: An Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Deas D, Brown ES. Adolescent substance abuse and psychiatric comorbidities. Journal of Clinical Psychiatry. 2006;67:e02. doi: 10.4088/jcp.0706e02. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Calabria B, Nelson P, Roberts A, Hall W, Lynskey M, Wiessing L, Mora ME, Clark N, Thomas J, Briegleb C, McLaren J GBD illicit drug use writing group. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug and Alcohol Dependence. 2011;117:85–101. doi: 10.1016/j.drugalcdep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Lynskey M, Hall W. Cohort trends in the age of initiation of drug use in Australia. Australia and New Zealand Journal of Public Health. 2000;24:421–426. doi: 10.1111/j.1467-842x.2000.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, Babor TF, Roebuck MC, Donaldson J. Changing the focus: The case for recognizing and treating cannabis use disorders. Addiction. 2002;97(Suppl 1):4–15. doi: 10.1046/j.1360-0443.97.s01.10.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Kendler KS, Prescott CA, Aggen SH, Gardner CO, Jr, Jacobson K, Neale MC. Longitudinal modeling of genetic and environmental in-fluences on self-reported availability of psychoactive substances: Alcohol, cigarettes, marijuana, cocaine and stimulants. Psychological Medicine. 2007;37:947–959. doi: 10.1017/S0033291707009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Current Opinion in Psychiatry. 2007;20:393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- Hall W, Lynskey M. Is cannabis a gateway drug: Testing hypotheses about the relationship between cannabis use and use of other illicit drugs. Drug and Alcohol Review. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- Hasin D, Paykin A. Alcohol dependence and abuse diagnoses: Concurrent validity in a nationally representative sample. Alcohol: Clinical and Experimental Research. 1999;23:144–150. [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. British Journal of Addiction. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley P, Bachman JG, Schu-lenberg J. [Accessed on 21st June, 2012];Marijuana use is rising; ecstasy use is beginning to rise; and alcohol use is declining among US teens. 2011 Retrieved from http://www.monitoringthefuture.org.
- Kandel D. Stages and pathways of drug involvement. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- Kandel D, Faust R. Sequence and stages in patterns of adolescent drug use. Archives of General Psychiatry. 1975;32:923–932. doi: 10.1001/archpsyc.1975.01760250115013. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Huang FY, Davies M. Comor-bidity between patterns of substance use dependence and psychiatric syndromes. Drug and Alcohol Dependence. 2001;64:233–241. doi: 10.1016/s0376-8716(01)00126-0. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. Journal for the Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journl of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: Re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Glowinski AL, Todorov AA, Bucholz KK, Madden PA, Nelson EC, Statham DJ, Martin NG, Heath AC. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Archives of General Psychiatry. 2004;61:1026–1032. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Lynskey M, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behavior Genetics. 2006;36:195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Lynskey M, White V, Hill D, Letcher T, Hall W. Prevalence of illicit drug use among youth: Results from the Australian School Students’ Alcohol and Drugs Survey. Australia and New Zealand Journal of Public Health. 1999;23:519–524. doi: 10.1111/j.1467-842x.1999.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Meyers JL, Dick DM. Genetic and environmental risk factors for adolescent-onset substance use disorders. Child and Adolescent Psychiatric Clinics of North America. 2010;19:465–477. doi: 10.1016/j.chc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral AR, McCafrey DF, Paddock SM. Evidence does not favor marijuana gateway effects over a common-factor interpretation of drug use initiation: Responses to Anthony, Kenkel & Mathios and Lynskey. Addiction. 2002;97:1509–1510. doi: 10.1046/j.1360-0443.2002.00297.x. [DOI] [PubMed] [Google Scholar]
- Neale MC. Statistical Modeling with Mx. Department of Psychiatry; Box # 980710, Richmond, VA 23298: 2004. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: Cohort study. BMJ. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Lieb R, Hofler M, Schuster P, Sonntag H, Wittchen HU. Patterns of cannabis use, abuse and dependence over time: Incidence, progression and stability in a sample of 1228 adolescents. Addiction. 1999;94:1663–1678. doi: 10.1046/j.1360-0443.1999.941116635.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS User Guide, Version 8.2. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Shelton K, Lifford K, Fowler T, Rice F, Neale M, Harold G, Thapar A, van den Bree M. The association between conduct problems and the initiation and progression of marijuana use during adolescence: A genetic analysis across time. Behavior Genetics. 2007;37:314–325. doi: 10.1007/s10519-006-9124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STATA Corp. STATA, Statistical Software. College Station, TX: STATA Corp; 2003. [Google Scholar]
- Teesson M, Dietrich U, Degenhardt L, Lynskey M, Beard J. Substance use disorders in an Australian community survey. Drug and Alcohol Review. 2002;21:275–280. doi: 10.1080/0959523021000002741. [DOI] [PubMed] [Google Scholar]
- Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: Exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. American Journl of Epidemiology. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Wagner FA, Anthony JC. Exposure opportunity as a mechanism linking youth marijuana use to hallucinogen use. Drug and Alcohol Dependence. 2002;66:127–135. doi: 10.1016/s0376-8716(01)00191-0. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Composite international diagnostic interview, Version 1.1. Researcher’s manual. Geneva: World Health Organization; 1994. [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems. 10. Geneva, Switzerland: World Health Organization; 2007. Revision. [Google Scholar]