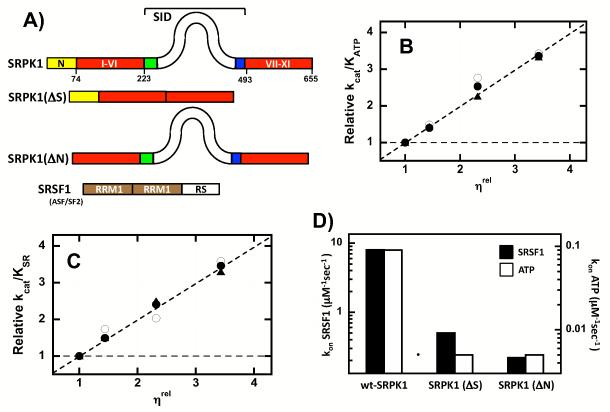
Figure 1. Diffusion Limits of ATP and SRSF1 in the Active Site of Wild-Type & Mutant Forms of SRPK1.
A) Enzyme & Substrate Constructs. B,C) Viscosity Effects on Steady-State Kinetic Parameters. Relative values for kcat/KATP (B), and kcat/KSR (C) in the absence and presence of varying sucrose concentrations are measured as a function of relative solvent viscosity (ηrel) for wild-type SRPK1 (○), SRPK1(ΔN) (•) and SRPK1(ΔS) (▲). Dotted lines reflect theoretical slope limits of 0 and 1. D) Bar graph showing changes in the association rate constants for SRSF1 and ATP.