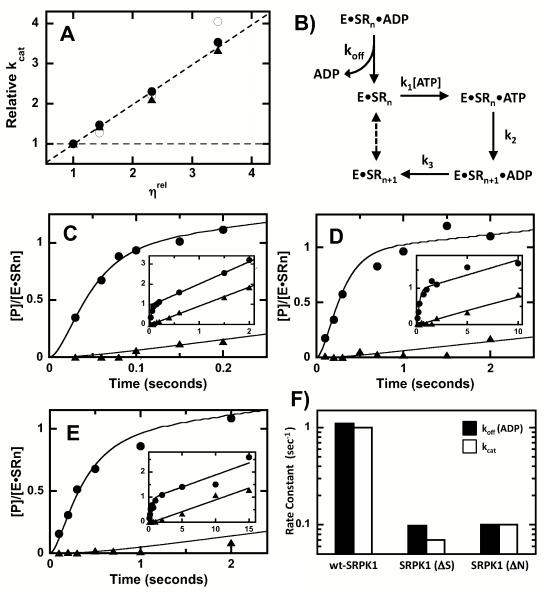
Figure 2. N-Terminus & SID Promote ADP Dissociation from SRPK1.
A) Viscosity effects on kcat for wild-type SRPK1 (○), SRPK1(ΔN) (•) and SRPK1(ΔS) (▲). Dotted lines reflect theoretical slope limits of 0 and 1. B) Kinetic Mechanism. Enzyme (E) and SRSF1 (SRn) are preequilibrated in the absence or presence of ADP and the reaction is started with ATP (k1). SRSF1 (SRn) is phosphorylated at the first site (k2), ADP is released (k3) and the product (SRn+1) becomes the substrate for the next phosphorylation event (dotted line). C-E) CATTRAP Experiments. SRSF1 and wild-type SRPK1 (C), SRPK1(ΔN) (D) and SRPK1(ΔS) (E) are prequilibrated in the absence (•) and presence (▲) of 120 ΔM ADP (in syringe) before the reaction is initiated with 600 ΔM 32P-ATP. Insets show extended time frames for the reactions (2-15 sec). The data are simulated to obtain values for koff, k2, k3 and [E*SRn] (Table 2). F) Bar graph showing changes in koff for ADP and kcat.