Fig. 1.
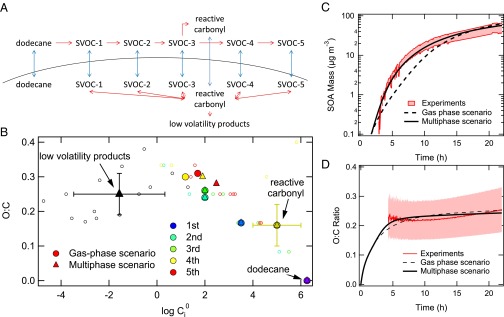
Modeling secondary organic aerosol formation from dodecane photooxidation. (A) Schematic of the mass transport (blue arrows) and chemical reactions (red arrows) in the dodecane SOA system. Multigeneration of SVOCs is considered in the gas phase. The multiphase scenario includes second-order reactions (red double arrows) between reactive carbonyl and SVOCs forming low-volatility products in the particle phase. (B) O:C ratios for products of dodecane oxidation vs. their predicted gas-phase saturation concentration (μg⋅m−3) over the pure (subcooled) liquids (Ci0). The smaller symbols indicate individual products predicted in the dodecane photooxidation chemical mechanism developed by Yee et al. (11). Color indicates the gas-phase generation in which compound is formed. These compounds include reactive carbonyl compounds (fourth generation). Black symbols indicate particle-phase products. The large solid circles and triangles indicate the surrogate SVOC compounds in the gas-phase and multiphase scenarios, respectively. Measured (red) and modeled SOA mass concentration (C) and O:C ratio (D) with the gas-phase (dashed line) and multiphase (solid line) scenarios, respectively. The upper and lower red lines in the C correspond to upper and lower bound wall loss corrections, respectively. The measured O:C ratio is subject to ±30% uncertainty (12).
