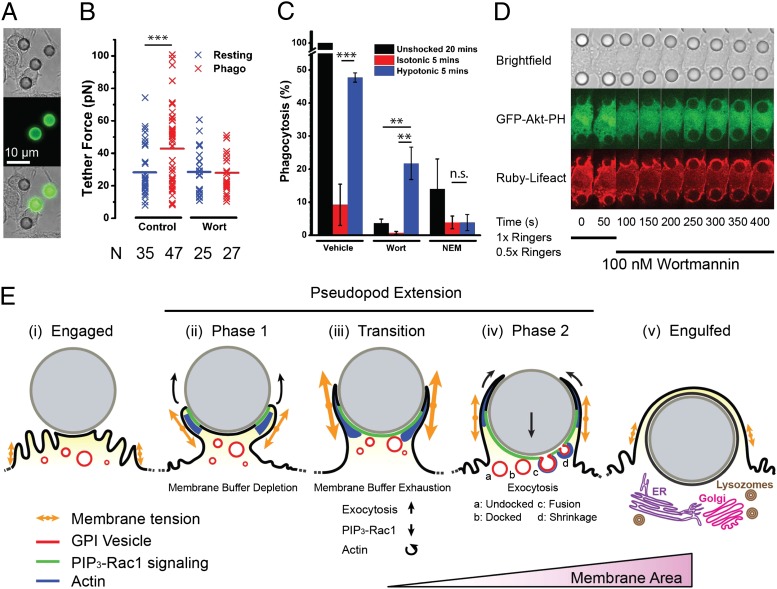
Fig. 4.
Phagocytosis of particles is promoted by increased membrane tension. (A) Transmitted light (Top), Alexa-488–anti-IgG (Middle), and overlay of macrophages (Bottom) ingesting 8-µm IgG-opsonised latex beads, fixed after 20 min. (B) Comparison of tether force measured in either resting cells or macrophages ingesting 8-µm beads in the presence or absence of 100 nM wortmannin. “N” indicates the number of data points in each condition. (C) Phagocytic efficiency (relative to 20-min ingestion) for cells without shock, with isotonic shock, or with hypotonic shock with or without wortmannin (100 nM) or NEM (2 µM). (D) Macrophages were incubated with wortmannin (100 nM) and exposed to 8-µm IgG-opsonised latex beads, resulting in stalled phagocytosis. After 50 s, a hypotonic shock (0.5× Ringer’s) was applied, initiating particle ingestion. (E) Model for pseudopod extension. After bead engagement (i), PIP3-Rac1 signaling is activated and actin polymerization pushes the pseudopod membrane forward around the particle during the first phase (ii). Membrane tension is higher than prior engagement because of protrusion, leading to membrane buffer (folds) depletion. At the transition (iii), a spike in membrane tension due to competition between membrane area depletion and protrusive activity leads to PIP3-Rac1 deactivation and actin reorganization in the pseudopod as well as exocytosis activation. In the second phase of pseudopod extension (iv), the membrane area is increased by an actin-associated exocytosis of GPI-anchored protein-containing vesicles. Particle internalization is initiated, and pseudopodia extension progressively wraps the particle as it enters the cell body. After engulfment (v), maturation is initiated and subsequently involves lysosome fusion and, potentially, Golgi and ER compartments.