Table 1.
Optimization of the Reaction Conditions a
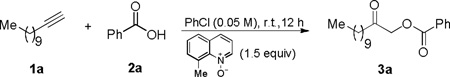 | |||
|---|---|---|---|
| entry | 1a/2a | catalyst | yieldb |
| 1 | 1:1.2 | LAuCl (5 mol %)/NaBArF4 (10 mol %) | <7 c |
| 2 | 1:1.2 | Me-DalPhosAuCl (5 mol %)/NaBArF4(10 mol %) | 11% |
| 3 | 1:1.2 | Mor-DalPhosAuCl (5 mol %)/NaBArF4(10 mol %) | 68% |
| 4 | 1:1.2 | L1AuCl(5 mol %)/NaBArF4(10 mol %) | 68% |
| 5 | 1:1.2 | L2AuCl(5 mol %)/NaBArF4(10 mol %) | 68% |
| 6 | 1:1.2 | L3AuCl(5 mol %)/NaBArF4(10 mol %) | 18% |
| 7 | 1:1.2 | L4AuCl(5 mol %)/NaBArF4(10 mol %) | 75% |
| 8 | 1:1.2 | L5AuCl(5 mol %)/NaBArF4(10 mol %) | 84% |
| 9 | 1:1.2 | L6AuCl(5 mol %)/NaBArF4(10 mol %) | 30% |
| 10 | 1:1.2 | L7AuCl(5 mol %)/NaBArF4(10 mol %) | 79% |
| 11 | 1:1.2 | L8AuCl(5 mol %)/NaBArF4(10 mol %) | 63% |
| 12 | 1.3/1 | L7AuCl(5 mol %)/NaBArF4(10 mol %) | 86% |
| 13 | 1.3/1 | L5AuCl(5 mol %)/NaBArF4(10 mol %) | 98% d |
| 14 | 1.3/1 | L5AuCl(5 mol %)/NaBArF4(10 mol %)e | 95% |
| 15 | 1.3/1 | L5AuCl(5 mol %)/NaBArF4(10 mol %)f | 88% |
The reaction was run with everything except the oxidant in a vial capped with a septum, and the oxidant was introduced into the reaction mixture in 12 h using a syringe pump. Initially, [1a] = 0.1 M.
Measured by 1H NMR analysis using diethyl phthalate as the internal standard.
L = Ph3P, IPr, or BrettPhos; the crude 1H NMR spectra were mostly messy.
96% isolated yield.
DCE was used as the solvent.
toluene was used as the solvent.
