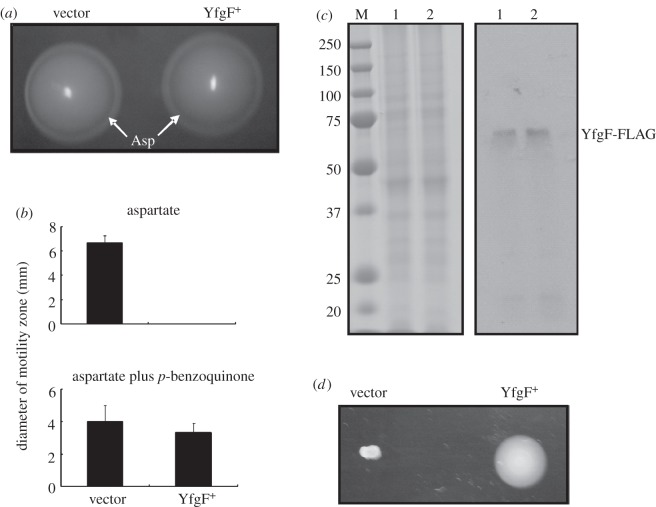
Figure 3.
YfgF-mediated inhibition of aspartate chemotaxis is relieved by exogenous p-benzoquinone. (a) Swimming motility of S. Enteritidis transformed with plasmids expressing no YfgF (vector control) and S. Enteritidis YfgF in the presence of p-benzoquinone (200 µg ml−1) under aerobic conditions. The aspartate (Asp) chemotactic ring (arrowed) is apparent in the absence (left) and presence (right) of YfgF. (b) The motility of S. Enteritidis was assessed on medium containing aspartate in the presence and absence of p-benzoquinone under aerobic conditions. In all cases, each experiment was performed a minimum of three times (mean values with standard deviation are shown). (c) Coomassie blue-stained SDS polyacrylamide gel (left) and Western blot developed with anti-FLAG antibodies (right) for S. Enteritidis transformed with pGS2421 (YfgF+) in the absence (lane 1) and presence (lane 2) of p-benzoquinone (200 µg ml−1). The Coomassie blue-stained gel shows the protein loading for each lane. The locations of the YfgF protein and the protein markers to calibrate the gel (lane M; molecular weights in kDa) are indicated. (d) Motility of E. coli yhjH mutant transformed with pGS2390 (vector control) or pGS2421 (YfgF+) in the presence of p-benzoquinone (200 µg ml−1). Plates were incubated under aerobic conditions for 20 h at 37°C.