Figure 5.
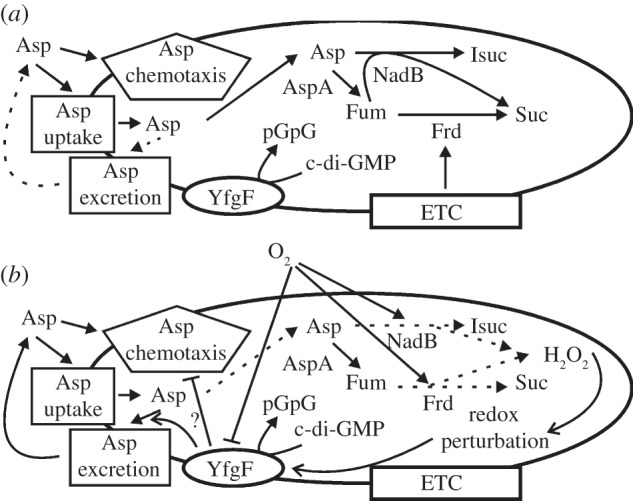
YfgF modulates aspartate chemotaxis to lessen oxidative damage when anaerobic bacteria are first exposed to molecular oxygen (O2). (a) In the absence of O2, expression of yfgF is maximal [3] and the proton motive force is low [24]. Under these conditions aspartate chemotaxis (in the presence of an appropriate energy source), aspartate uptake and metabolism are supported (solid arrows). Aspartase (AspA) converts Asp to fumarate (Fum), which in turn is reduced to succinate (Suc) by fumarate reductase (Frd) using reducing equivalents from the electron transport chain (ETC). Aspartate is also the substrate for NadB, which converts Asp to iminosuccinate (Isuc), which is required for NAD+ biosynthesis, with Fum acting as the electron acceptor [25]. (b) If left unchecked, when anaerobic bacteria are exposed to O2 NadB and Frd use O2 as an electron acceptor [22,23], generating the reactive oxygen species hydrogen peroxide (H2O2) (broken arrows), resulting in oxidative stress and redox perturbation. It is suggested (denoted by question mark) that the MASE1 domain of YfgF perceives the onset of this stress and either inhibits (⊥) aspartate chemotaxis or promotes (↑) aspartate excretion to decrease Asp utilization and thereby limit H2O2 generation until the bacteria have adapted to the new aerobic conditions. The presence of O2 switches off the transcription factor FNR, thereby minimizing yfgF expression (⊥). After adaptation growth and protein turnover removes YfgF from the now aerobic bacteria, restoring aspartate utilization and observable aspartate chemotaxis. Sustained expression of yfgF in the experimental system used here has allowed this normally transient behaviour to be revealed. Hydrolysis of c-di-GMP by YfgF promotes motility under anaerobic and aerobic conditions.
