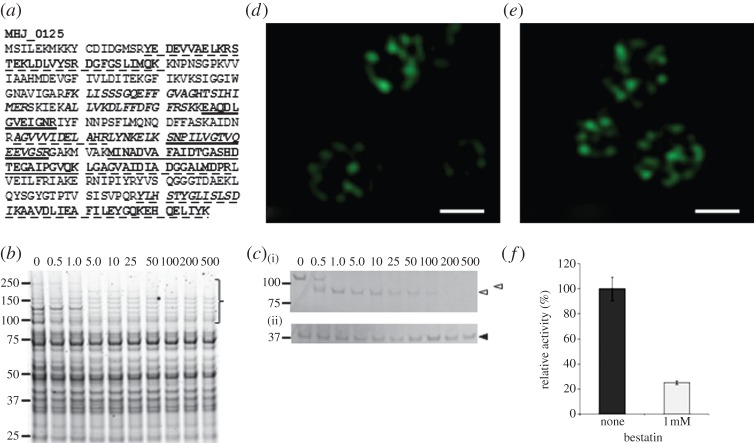
Figure 1.
MHJ_0125 is a surface-accessible and secreted protein. (a) Tryptic peptides matching to MHJ_0125 from surface proteome analyses. Peptides in italics were identified by shaving freshly cultured M. hyopneumoniae cells with trypsin. Peptides underscored with a dashed line correspond to tryptic peptides of biotinylated MHJ_0125 recovered by avidin chromatography. Single underlined peptides were generated by digesting M. hyopneumoniae proteins released in PBS (secreteome) with trypsin. Double underscored tryptic peptides were common in preparations derived from avidin chromatography and secreteome studies. (b) Cell lysates of M. hyopneumoniae cells exposed to different concentrations of trypsin (0–500 µg ml−1 at 37°C for 15 min). Protein profiles indicate that the cell membrane remains intact under these experimental conditions. (c) Western blot of M. hyopneumoniae lysates from cells exposed to different concentrations of trypsin probed with rabbit anti-P97 N-terminal serum (i) and anti-MHJ_0125 serum (ii). The P97 cilium adhesin (top empty arrow) is reported to be highly sensitive to trypsin [17] and is recognized by anti-P97 N-terminal antibodies. MHJ_0125 was not completely degraded by trypsin because of its presence inside the cell cytosol. (d,e) Structured illumination microscopy images of M. hyopneumoniae cells probed with rabbit anti-MHJ_0125 followed by goat anti-rabbit HRP conjugated to Alexa Fluor 488. Fixed, non-permeabilized cells (d) show MHJ_0125 only on the cell surface, whereas permeabilized, fixed cells (e) show MHJ_0125 is also present inside the cell. Images were taken on an OMX Deltavision microscope. Scale bar, 0.5 µm. (f) Cell surface-associated glutamyl aminopeptidase activity was confirmed by culturing live M. hyopneumoniae in the presence of the fluorescent substrate H-Glu-AMC, with or without the aminopeptidase inhibitor bestatin. Data are shown as the relative enzyme activity, expressed as a percentage against no bestatin (none) that is taken to be 100% activity. Data represent mean fluorescence units from three independent assays±s.d.