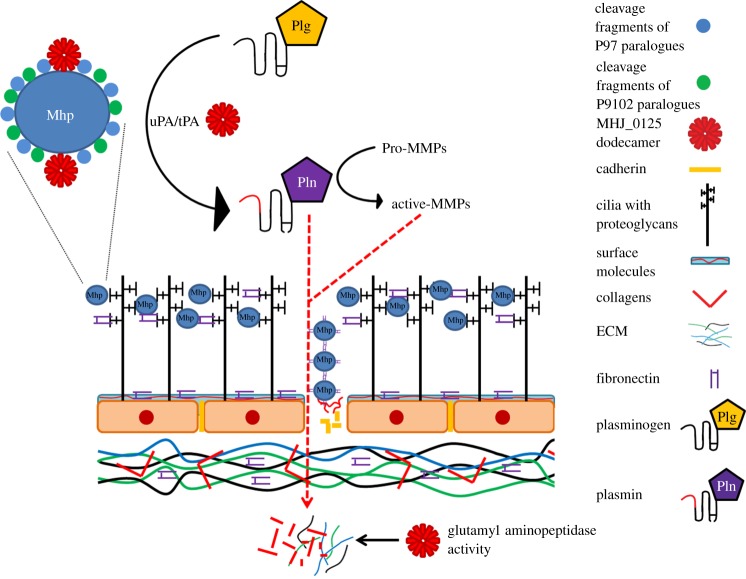
Figure 12.
Interaction of M. hyopneumoniae with the porcine respiratory epithelium. P97 and P102 adhesin families bind glycosaminoglycans and fibronectin on the surface of porcine cilia. P97 and P102 adhesins and MHJ_0125, shown here as a dodecamer, bind porcine plasminogen and induce conformational changes that facilitate its conversion, on the M. hyopneumoniae cell surface, to plasmin by tPA and urokinase-type plasminogen activator (uPA). Plasmin is a broad spectrum serine protease that degrades host extracellular matrix (ECM) and other host proteins, and activates matrix metalloproteases (MMPs) that further breakdown ECM. Cilial and epithelial cell damage initiated by infection by M. hyopneumoniae stimulates the expression and secretion of fibronectin. The combined activity of plasmin and MMPs generates substrates for MHJ_0125 and other putative proteases on the surface of M. hyopneumoniae. Surface-bound plasmin targets cadherin located in intercellular junctions which presumably allows M. hyopneumoniae to gain entry to subepithelial sites.