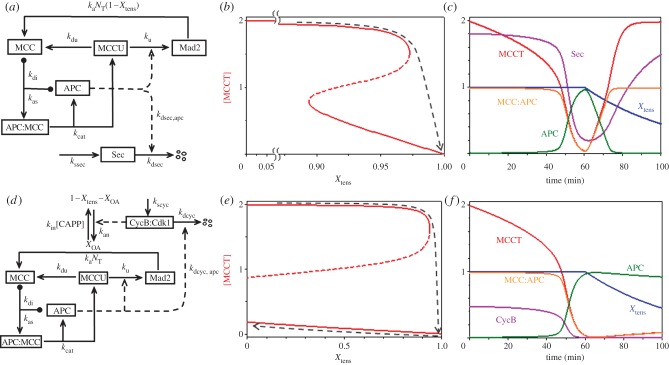
Figure 5.
The mitotic checkpoint. (a) Wiring diagram. The mitotic checkpoint complex (MCC) binds to and inhibits the anaphase-promoting complex (APC). APC ubiquitinates MCC, and the ubiquitin moiety is removed by a de-ubiquitinase. Poly-ubiquitination of MCC leads to degradation of some of its components (notably Cdc20) and release of inactive checkpoint proteins (labelled by Mad2 only). Checkpoint proteins (Mad2, etc.), are reactivated by tensionless centromeres, N0 = NT(1 − Xtens), leading to reassembly of MCC. Securin degradation by active APC leads to dissolution of cohesin complexes and separation of sister chromatids in early anaphase. (b) Signal–response curve: [MCCT], the total concentration of active MCC, versus Xtens, the fraction of centromeric regions that are in tension on the mitotic spindle. The black dashed curve indicates that the mitotic checkpoint is engaged (i.e. [MCCT] large) until the last chromosome aligns on the mitotic spindle. When Xtens increases above approximately 0.97, the checkpoint disengages and APC is activated. As the cell enters anaphase, Xtens drops back to zero. (c) Simulation of in vitro release of the mitotic checkpoint. Compare with fig. 1 of Reddy et al. [23]. Note that when Xtens drops below approximately 0.89 the checkpoint re-engages, as predicted by the signal-response curve in (b). (d) Wiring diagram of the CycB–MCC–APC network. Cycin B-dependent kinase is required for activation of tensionless centromeres, X0A. (e) Signal–response curve: [MCCT] versus Xtens. Now the threshold for re-engaging the checkpoint has moved to negative values of Xtens, and the checkpoint remains disengaged when Xtens drops to zero in anaphase (black dashed line). (f) Simulation of in vivo release of the mitotic checkpoint. The checkpoint mechanism does not re-engage as Xtens decreases.