Abstract
During a study of the helminth fauna of 1,643 rodents trapped along the Mekong River (Thailand, Lao People’s Democratic Republic and Cambodia) in 2008–2011, the spirurid nematode Physaloptera ngoci Le-Van-Hoa, 1961 was recovered with an overall prevalence of 2.8%. Based on the original description, it was identified in nine of 23 different Murinae host species and is here reported for the first time from these three countries. A scanning electron microscopy study provides additional morphological data.
Keywords: Helminths, Physaloptera ngoci, Rodents, Thailand, Lao People’s Democratic Republic, Cambodia
Abstract
Lors de l’étude des helminthes de 1,643 rongeurs, capturés le long du Mékong en Thaïlande, République Démocratique Populaire du Laos et Cambodge entre 2008 et 2011, le nématode Spiruridae Physaloptera ngoci a été détecté avec une prévalence globale de 2.8 %. En accord avec la description originale, P. ngoci a été identifié chez neuf des 23 espèces de Murinae étudiées et est signalé pour la première fois dans ces trois pays. Une étude en microscopie électronique à balayage apporte des nouvelles données morphologiques.
Introduction
Unnamed species of Physaloptera Rudolphi, 1819 were reported from South-east Asia (SEA) in rodents belonging to the family Muridae on a number of occasions. In West Java (Indonesia), Physaloptera sp. was recorded in Maxomys bartelsii (as Rattus bartelsii) and Niviventer lepturus (as Rattus lepturus) [16]; in the same area, Hasegawa et al. [4] found specimens of Physaloptera in Rattus argentiventer. In Malaysia, immature stages of Physaloptera sp. were reported in seven different rodent species: Berylmys bowersi, Leopoldamys sabanus, Maxomys rajah, R. argentiventer (as Rattus rattus argentiventer), Rattus tiomanicus (as Rattus jalorensis and Rattus rattus rumpia), Sundamys mulleri (as Rattus mulleri) [15] and Rattus sp. [11]. Unnamed specimens of Physaloptera were also reported from Asia outside SEA, namely from Japan in Apodemus agrarius and Rattus rattus [3] and from India in R. rattus (as Rattus rattus rufescens) and Tatera indica (as Tatera indica indica) [10].
Physaloptera species were also reported from the SEA region in mammals of the family Sciuridae, specifically Physaloptera inermis Linstow, 1906 in Callosciurus prevostii (as Sciurus prevostii) from Malaysia and Physaloptera sciuri Parona, 1898 in Callosciurus melanogaster (as Sciurus melanogaster) from Indonesia [5, 13]. In the Indian region (close to SEA) Physaloptera funambuli Parihar & Nama, 1978 was detected in Funambulus pennanti [10, 12].
Bearing the above-mentioned undetermined species in mind, Physaloptera ngoci is to date the only known species of this genus in Muridae from SEA. Physaloptera ngoci was also described from Vietnam in Rattus norvegicus [5] and subsequently recorded in Mus sp. and Rattus sp. [14].
In the present study, rodents were captured in SEA during 2008–2011 and their helminths, including P. ngoci, recovered.
Materials and methods
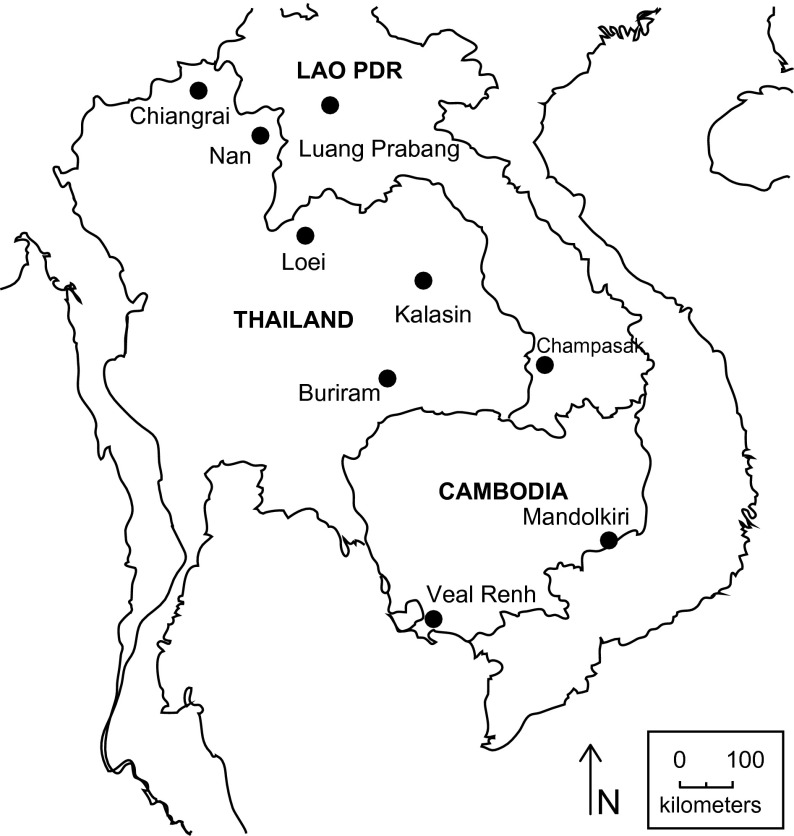
Murid hosts were captured at nine different sampling sites along the Mekong River in Thailand, the Lao People’s Democratic Republic (PDR) and Cambodia (Figure 1). The rodents were trapped with baited traps, either locally made or Sherman. At each sampling locality, ten trap lines (composed of ten traps, placed every five metres) were set over a period of 12 nights. Trap lines were moved every four nights. Complementary trapping was carried out in villages and isolated houses, with five traps per house. Rodents were identified by morphology or using species-specific primers and/or barcoding assignments (details available in the Barcoding Tool/RodentSEA section of the CERoPath project website: www.ceropath.org). Rodents were euthanized and dissected following protocols that maximize animal care, the health and safety of field parasitologists and the generation of quality data.
Figure 1.
Location of nine CERoPath sampling sites in South-east Asia.
Viscera were examined under a stereomicroscope and helminth parasites were collected and preserved in 70% ethanol. Nematodes were cleared in lactophenol and hand-cut sections of the male anterior end were mounted en face for identification. Females were dissected in order to count the number of uteri. Photographs and measurements were taken with a microscope mounted on a Tucsen camera with associated software. Measurements taken from 10 adult individuals of each sex (males from Bandicota berdmorei, Bandicota indica and Bandicota savilei and females from B. berdmorei, B. indica and R. argentiventer) are given in micrometres unless otherwise specified and listed as range and median.
For Scanning Electron Microscopy (SEM) studies, nematodes were fixed in 70% ethanol, critical point-dried, mounted on SEM studs and finally coated with a thin layer of platinum before being examined with a Zeiss DSM 940A Scanning Electron Microscope at an accelerating voltage of 15 kV.
A subset of studied specimens was deposited in the Museu de Ciències Naturals de Barcelona, Catalonia, Spain, with Accession Numbers MZB 2013-0019 and MZB 2013-0020 (two females), and MZB 2013-0021 and MZB 2013-0022 (two males). Murid hosts were identified following the nomenclature and classification in Chaval [1].
Results and discussion
A total of 1,643 rodents belonging to 23 species were examined and in nine of these species a nematode belonging to the genus Physaloptera was isolated from the stomach and identified as P. ngoci (details of hosts and prevalence are summarized in Table 1). The general prevalence was 2.8%, the highest prevalence being 40% in R. argentiventer (n = 10) from Veal Renh, Cambodia. Physaloptera ngoci was absent in the following rodents: B. bowersi (n = 27), Chiropodomys gliroides (n = 2), Hapalomys delacouri (n = 1), Leopoldamys edwardsi (n = 14), L. neilli (n = 1), Maxomys surifer (n = 81), Mus cookii (n = 141), M. fragilicauda (n = 1), Mus sp. (n = 3), Niviventer fulvescens (n = 76), Rattus andamanensis (n = 1), R. nitidus (n = 14) and R. norvegicus (n = 17).
Table 1.
Prevalence (%) of Physaloptera ngoci and number of rodents (n) examined at study sites in Thailand, Lao People’s Democratic Republic and Cambodia (localities within the same country are pooled).
| Hosts | Thailand | Lao | Cambodia | Overall (n = 1,643) |
|---|---|---|---|---|
| Bandicota indica | 6.2% (n = 177) | 0% (n = 3) | 0% (n = 1) | 6.1% (n = 181) |
| Bandicota savilei | 13.6% (n = 22) | 100% (n = 1) | 8% (n = 50) | 11.0% (n = 73) |
| Berylmys berdmorei | 5.3% (n = 19) | 0% (n = 3) | 0% (n = 6) | 3.6% (n = 28) |
| Mus caroli | 1.3% (n = 76) | 0% (n = 39) | 0% (n = 1) | 0.9% (n = 116) |
| Mus cervicolor | 0.8% (n = 130) | – | – | 0.8% (n = 130) |
| Rattus argentiventer | 0% (n = 7) | – | 40% (n = 10) | 23.5% (n = 17) |
| Rattus exulans | 0.5% (n = 214) | 0% (n = 50) | 1.5% (n = 67) | 0.6% (n = 331) |
| Rattus sakeratensis | 10.9% (n = 92) | 10% (n = 10) | – | 10.8% (n = 102) |
| Rattus tanezumi | 0% (n = 134) | 0% (n = 90) | 11.3% (n = 62) | 2.5% (n = 286) |
| Total (n = 1,643) | 2.6% (n = 1074) | 0.7% (n = 287) | 5.7% (n = 282) | 2.8% (n = 1643) |
The observation of structures in the cephalic region, the distribution of the cephalic papillae, the arrangement and number of male caudal papillae, the size of the male spicules, the number of female uteri, the total length and the distance from the anterior end to the vulva all confirm the identification of this helminth as P. ngoci, as per the original description [5].
The cephalic region (see Figure 2A–F) bears two semicircular pseudolips, each bearing a pair of papillae, one amphid, a single externolateral tooth and a trident-like internolateral tooth. The arrangement of the 21 caudal papillae in males is as follows: (i) four pairs of pedunculate papillae (one pair precloacal, one adcloacal and two postcloacal) and three single sessile papillae, distributed as a precloacal pair and a single adcloacal papilla; (ii) two pairs of sessile, immediately postcloacal papillae and (iii) four pairs of sessile papillae located on the tail, distributed in two groups of two pairs, one group towards the middle and the other nearer the end of the tail (Figure 3). Left spicule 370–574 (470) and right spicule 259–354 (296) long. Female total length 17.92–28.74 (21.17) mm. Distance from the anterior end to the vulva 2.06–4.58 (3.48) mm. Vulva in the first third of the body. Four uteri. Eggs 23.6–33.3 (28.1) × 38.4–48.6 (44.5).
Figure 2.
Physaloptera ngoci. A, cephalic extremity, general view. B, cephalic extremity, lateral view, cephalic collarette (CC). C, cephalic extremity with detail of papillae (P), lateral view. D, cephalic extremity, apical view. AP, amphids; PL, porus-like region; P, papillae. E, F, detail of externolateral tooth (ELT) and tripartite internolateral tooth (ILT).
Figure 3.
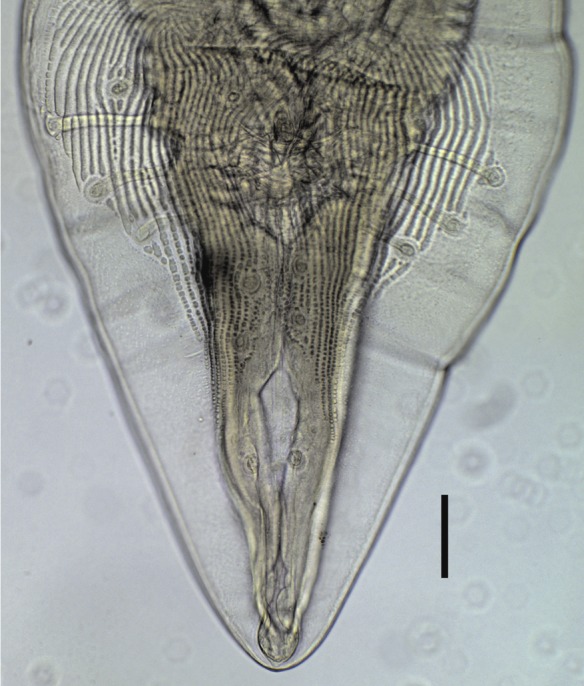
Physaloptera ngoci male. Caudal papillae, ventral view. Scale bar: 100 μm.
The morphology of our samples confirms the identification of this nematode as P. ngoci given the similarity of structures and their disposition in the cephalic region, the same number and similar arrangement of male caudal papillae, and the same number of uteri in the females. In addition, our measurements match those provided in the original description: left and right spicule 460 and 300 long, respectively, total length 25 mm in females, distance from the anterior end to the vulva 3.32 mm and egg size 28 × 45 [5].
Of the congeners previously recorded from small mammals in India and the SEA region, all share the presence of four uteri in the females, but Physaloptera funambuli differs from P. gnoci in the number of male caudal papillae (10 pairs), a much longer left spicule 2.72–2.88 (2.80) mm and a shorter right spicule 0.19–0.20 (0.193) mm [12]. The total female length is greater 27.55–49.20 (35.12) mm [12], as is the distance from the anterior end to the vulva 4.67–9.26 (5.88) mm [12]. Eggs are smaller 20–40 (24) × 20–45 (28) [12].
Physaloptera inermis differs from P. gnoci in the number of male caudal papillae (eight pairs) and longer spicules 2.37 mm [6] (no differences between right and left spicule specified). The total female length is greater 51 mm [6]. The distance from the anterior end to the vulva is not given in the original description. Egg size is similar 26 × 47 [6].
Physaloptera sciuri differs in the number (15) and disposition of male caudal papillae (no sessile precloacal papillae, one unpaired sessile postcloacal papilla and the final pair of postcloacal papillae close to the tail). Spicule lengths are not provided in the original description. The total female length 17–32 mm is similar [13]. The vulva is approximately halfway down the body and we were clearly able to differentiate its position from that in P. gnoci, in which it is more anteriorly. The eggs are smaller 16 × 22 [13].
SEM studies are currently available for four species of Physaloptera: Physaloptera sibirica Petrow & Gorbunow, 1931 in mammals from Spain [9]; Physaloptera bispiculata Vaz & Pereira, 1935 in the South American water rat Nectomys squamipes from Brazil [8]; Physaloptera sp. in an anuran from Argentina [2]; and Physaloptera herthameyerae Lopes et al., 2009 in a marsupial from Brazil [7]. The present study increases the number of SEM studies in Physaloptera to five and is the first such study done on a species from hosts in SEA.
This is the first report of P. ngoci from rodents in Cambodia, Lao PDR and Thailand. While its original description was based on specimens from a single host species, the brown rat (R. norvegicus) from Vietnam, several new hosts are listed for P. ngoci in the present study. Its host spectrum now includes several species of the subfamily Murinae; likewise, its known geographic range is expanded to include SEA. Previous reports of Physaloptera spp. in SEA murine rodents can thus probably be assigned to P. ngoci given that our data confirm that this nematode is widespread in SEA.
Acknowledgments
This study was supported by French ANR 11 CPEL 002, project BiodivHealthSEA, Local impacts and perceptions of global changes: Biodiversity, health and zoonoses in Southeast Asia (www.biodivhealthsea.org) and the French ANR Biodiversity ANR 07 BDIV 012, project CERoPath, Community Ecology of Rodents and their Pathogens in a changing environment (www.ceropath.org). A. Ribas and J. Miquel were partially supported by 2009-SGR-403 from the Catalan Government (Spain). We are especially grateful to Nuria Cortadellas and Almudena Garcia from the Unitat de Microscopia, Facultat de Medicina, Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB) for their help with the preparation of the samples.
Cite this article as: Veciana M, Chaisiri K, Morand S, Miquel J & Ribas A: New biogeographical and morphological information on Physaloptera ngoci Le-Van-Hoa, 1961 (Nematoda: Physalopteridae) in South-east Asian rodents. Parasite, 2013, 20, 23.
References
- 1.Chaval Y. 2011. South East Asian Murines Field Guide. Thailand: ANR BiodivhealthSEA; p. 199 [Google Scholar]
- 2.Gonzalez CE, Hamann MI. 2010. First report of nematode parasites of Physalaemus santafecinus (Anura: Leiuperidae) from Corrientes, Argentina. Revista Mexicana de Biodiversidad, 81, 677–687 [Google Scholar]
- 3.Hasegawa H, Arai S, Shiraishi S. 1993. Nematodes collected from rodents on Uotsuri Island, Okinawa, Japan. Journal of the Helminthological Society of Washington, 60, 39–47 [Google Scholar]
- 4.Hasegawa H, Shiraishi S, Rochman. 1992. Tikusnema javaense n. gen., n. sp. (Nematoda: Acuarioidea) and other nematodes from Rattus argentiventer collected in west Java, Indonesia. Journal of Parasitology, 78, 800–804 [PubMed] [Google Scholar]
- 5.Le-Van-Hoa. 1961. Étude d’un nouveau Physaloptère du rat, trouvé au Viet-Nam. Annales de Parasitologie Humaine et Comparée, 36, 672–676 [PubMed] [Google Scholar]
- 6.Linstow O. 1906. Nematoden des zoologischen Museums in Königsberg. Archiv für Naturgeschichte, 72, 249–258 [Google Scholar]
- 7.Lopes Torres EJ, Maldonado A, Lanfredi RM. 2009. Spirurids from Gracilinanus agilis (Marsupialia: Didelphidae) in Brazilian Pantal wetlands with a new species of Physaloptera (Nematoda: Spirurida). Veterinary Parasitology, 163, 87–92 [DOI] [PubMed] [Google Scholar]
- 8.Mafra AC, Lanfredi RM. 1998. Reevaluation of Physaloptera bispiculata (Nematoda: Spiruroidaea) by light and scanning electron microscopy. Journal of Parasitology, 84, 582–588 [PubMed] [Google Scholar]
- 9.Miquel J, Segovia JM, Feliu C, Torres J. 1996. On Physaloptera sibirica Petrow et Gorbunov, 1931 (Nematoda: Physalopteridae) parasitizing Iberian mammals. Wiadomosci Parazytologiczne, 42, 435–442 [PubMed] [Google Scholar]
- 10.Nama HS. 1984. Faunistic survey of helminth parasites of vertebrates of Rajasthan with special reference to arid zone. Scientific Reviews on Arid Zone Research, 2, 187–225 [Google Scholar]
- 11.Paramasvaran S, Sani RA, Hassan L, Hanjeet K, Krishnasamy M, John J, Santhana R, Sumarni MG, Lim KH. 2009. Endo-parasite fauna of rodents caught in five wet markets in Kuala Lumpur and its potential zoonotic implications. Tropical Biomedicine, 26(1), 67–72 [PubMed] [Google Scholar]
- 12.Parihar A, Nama HS. 1978. Physaloptera funambuli sp. n. (Nematoda, Physalopteridae) from Funambulus pennanti. Current Science Bangalore, 47, 832–834 [Google Scholar]
- 13.Parona C. 1898. Elminti raccolti dal Dott. Elio Modigliani alle isole Mentawei, Engano e Sumatra. Annali del Museo civico di storia naturale Giacomo Doria, Serie 2, 19(39), 102–124 [Google Scholar]
- 14.Phan TV. 1984. The nematodes parasitizing on animals in Taynguyen Plateau. Tap Chi Sinh Hoc, 8, 22–29 [Google Scholar]
- 15.Singh M, Chee-Hock C. 1971. On a collection of nematode parasites from Malayan rats. Southeast Asian Journal of Tropical Medicine and Public Health, 2, 516–522 [PubMed] [Google Scholar]
- 16.Wiroreno W. 1978. Nematode parasites of rats in West Java, Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health, 9, 520–525 [PubMed] [Google Scholar]