Figure 1. Pathways implicated in contributing to mitochondrial dysfunction in the heart with aging.
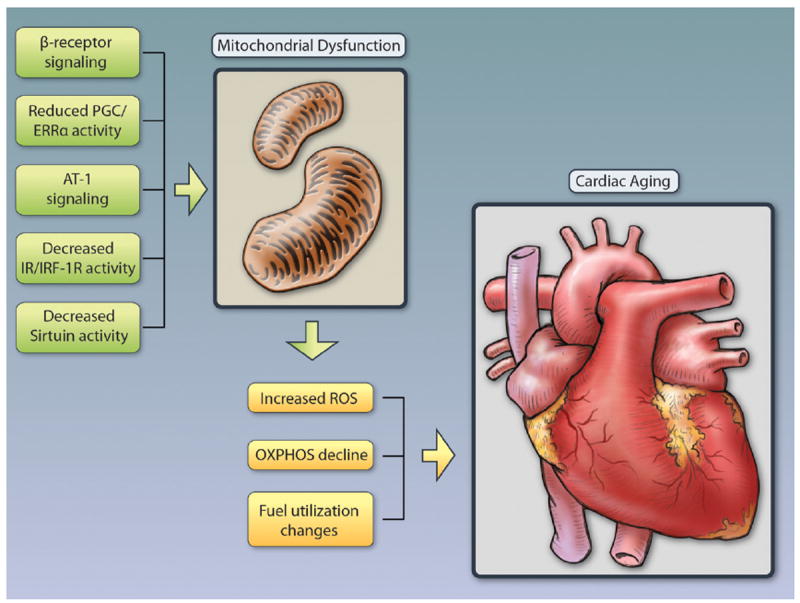
Multiple signaling pathways have been implicated in driving mitochondrial decline with age. Besides reduced activity of the PGC/ERRα network (see text) Angiotensin II has been suggested to play a role as activation of the Angiotensin receptor type I (AT-1) impairs mitochondrial biogenesis and mitochondrial respiratory chain activity and deletion of the AT-1 in mice is associated with increased number of mitochondria, decreased ROS induced oxidative damage, and improved cardiac function.117 Elevated norepinephrine and activation of β-adrenergic receptors in the heart increases ROS, impairs mitochondrial function and deletion of AC5 (the predominant isoform of adenyl cyclase in the heart), and protects from oxidative stress through activation of the RAF/MEK/ERK signaling pathway.118 Similarly, β-receptor blockade with carvedilol reduces oxidative stress levels in the heart.119,120 The insulin/IGF-1 pathway has been suggested to play a protective role, based on findings in mice deficient for either the insulin (CIRKO), the insulin-like growth factor 1 (IGF-1), or both receptors (MI2RKO).121,122 These mice have varying degrees of reduced oxidative phosphorylation with decreased ATP generation, increased ROS, reduced tricarboxylic acid cycle and fatty acid oxidation genes, and impaired cardiac function.123 Of the 7 mammalian sirtuins, Sirtuin 1 and Sirtuin 3 have been particularly implicated in delaying cardiac aging and protecting from oxidative stress. Illustration credit: Cosmocyte/Ben Smith.
