Figure 3. A unified theory of ageing.
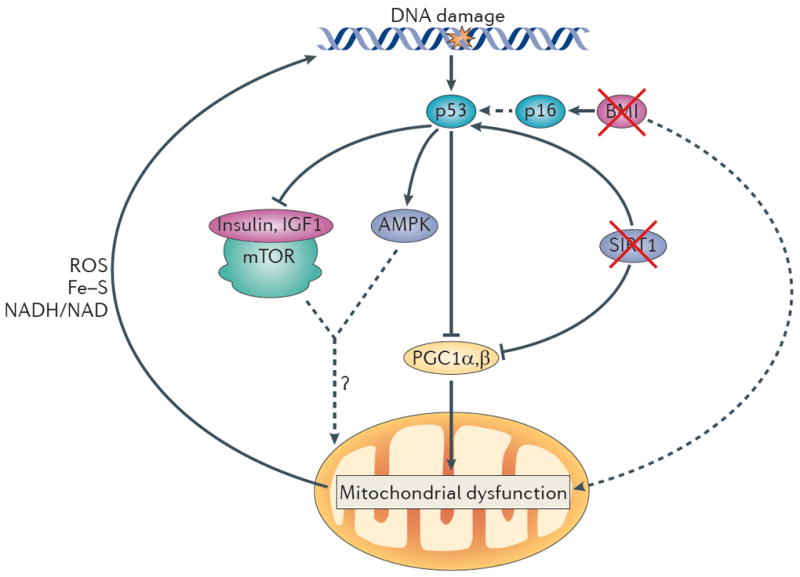
In this model, increased DNA damage (for example, owing to telomere attrition, impaired DNA repair and increased reactive oxygen species (ROS) levels) activates p53, and increasing levels of p53 ultimately lead to compromised mitochondrial function through the repression of PPARγ co-activator 1α (PGC1α) and PGC1β (which promote mitochondrial biogenesis). This p53-mediated mitochondrial dysfunction triggers a cycle of DNA damage (by affecting the production of ROS, iron–sulphur (Fe–S) clusters and NADH/NAD), which in turn leads to further p53 activation and mitochondrial compromise. This feed-forward loop could also account for the divergent and opposite effects of many players in the ageing process. Under mild stress conditions, several components depicted here (p53, mitochondria and AMP-activated protein kinase (AMPK)) have been shown to preserve cellular function but to promote cellular ageing under more severe stress conditions (see text). The interplay between p53 and other pathways that have been implicated in ageing is also indicated. p53 represses the activity of the insulin and insulin-like growth factor 1 (IGF1) pathway and the mammalian target of rapamycin (mTOR) pathway and activates AMPK. How the altered activity of these pathways modifies mitochondrial function and the ageing process in the setting of increased DNA damage is not clear. Other p53-dependent and p53-independent pathways might cooperate in inducing mitochondrial dysfunction. For example, BMI1 indirectly inhibits p53 activation, and BMI1 loss upregulates p16 expression, which increases p53 activity indirectly (dashed arrow) by interacting with MDM2, the negative regulator of p53 (not shown). BMI1 has also been shown to induce mitochondrial dysfunction (probably indirectly). In addition, loss of sirtuins may contribute to mitochondrial dysfunction, as active sirtuin 1 (SIRT1) decreases p53 activity, and loss of SIRT1 promotes p53 activation and its downstream functions. SIRT1 also activates PGC1α and thereby boosts mitochondrial biogenesis. The consequences of mitochondrial dysfunction are, when mild, functional impairment (for example, decreased ATP generation and β-oxidation) without cell loss. However, under increased stress conditions, mitochondrial dysfunction leads to functional impairment and concomitant loss of parenchymal mass owing to increased apoptosis and senescence.
