Abstract
Purpose
Treatment-related symptom burden varies significantly among patients undergoing radiotherapy or chemoradiotherapy, yet such variation is typically not reflected in the results from single-group studies. We applied group-based trajectory modeling (GBTM) to describe the heterogeneity of symptom burden among patients with head and neck cancer and to identify subgroups with distinct symptom development trajectories.
Methods
Patients (n = 130) were recruited pretherapy, and rated multiple symptoms weekly for 10 weeks via the M. D. Anderson Symptom Inventory. With the mean of five most-severe symptoms over time as an outcome measure, GBTM was used to identify patient subgroups with distinct symptom trajectories. Linear mixed-effects modeling (LMM) was applied to compare with GBTM’s ability to describe the longitudinal symptom data.
Results
The five most-severe symptoms were: problems with taste, difficulty swallowing or chewing, problems with mucus, fatigue, and dry mouth. A two-group GBTM model identified 68% of patients as having high symptom burden, associated with older age, worse baseline performance status, and chemoradiotherapy treatment. A four-group GBTM model generated one stable group (4% of patients) and three groups varying in symptom severity with both linear and quadratic functions over time. LMM revealed symptom-change patterns similar to that produced by GBTM but was inferior in identifying risk factors for high symptom burden.
Conclusions
For cancer patients undergoing aggressive therapy, GBTM is capable of identifying various symptom-burden trajectories and provides severity groupings that will aid research and may be of clinical utility. These results may be generalizable to other cancer types and treatments.
Keywords: symptom burden, group-based trajectory model, head and neck cancer, MDASI
Introduction
Management of locoregional advanced head and neck cancer (HNC) has increasingly relied on radiotherapy, with or without concurrent chemotherapy [1]. In clinical observation, patients with HNC have substantial individual differences in the severity of their treatment-related symptoms, even when disease severity and treatment modality are comparable. For example, in a cross-sectional study, Rosenthal et al. [2] reported that HNC patients could be grouped as those with either high or low symptom severity at the end of chemoradiotherapy or radiotherapy. Understanding this heterogeneity is a necessary step in characterizing high symptom burden that may result in treatment termination or interruption and for identifying factors that may contribute to high symptom burden.
Cluster analysis is the method most often used for cross-sectional studies of the heterogeneity of symptom burden [2–4]. We used a two-step cluster method to categorize a sample of patients with cancer into high-symptom and low-symptom groups one year after cancer diagnosis [5]. Using group (i.e., high symptom, low symptom) membership as an outcome variable, we found that poor socioeconomic status, age younger than 55 years, and the presence of more comorbid conditions were risk factors for higher symptom burden. Latent class analysis, a model-based cluster-analysis method, has also been applied to identify subgroups of patients [6]. Dodd et al. [7] divided cancer patients into four subgroups according to their ratings of pain, fatigue, sleep disturbance, and depression reported on the day of first dose of biotherapy. Further analysis showed that the subgroup with the most-severe symptoms had significantly lower functional status and poorer quality of life. These reports demonstrate the utility of these cluster-analysis methods for describing the heterogeneity of symptom burden among cancer patients, for identifying predictors for subgroups with high symptom burden, and for associating symptoms with other outcomes.
However, cluster analysis is designed for cross-sectional data, and latent class analysis is seldom used to describe longitudinal data collected at more than two time points due to its complexity in application. Because symptoms change over the course of cancer treatment, patient groupings may vary across time points. For example, Dodd et al. [7] identified four subgroups at the start of biotherapy but only three subgroups in the same patient population one month later. This inconsistent group membership across time may hinder further analysis on predictor identification and make it difficult to profile the developmental trajectories of patients’ symptom burden.
Several longitudinal models, such as the linear mixed-effect model (LMM) [8] and the hierarchical linear model [9], have been applied to describe the average developmental tendencies of cancer-related symptoms over time. An assumption underlying these methods is that patients have similar responses to accumulated treatment dose, resulting in a similar symptom experience over time. With these methods, variations typically are described in terms of covariates of interest. For instance, in a study of women with breast cancer, fatigue trajectories were described by different levels of age, body mass index, and disturbed sleep [10]. However, an external categorization criterion used to construct subgroups of developmental trajectories must be based on an assumed prior, usually patients’ demographic information or clinical characteristics. In most cases, currently identified predictors can explain only a small part of the variability. Klepstad et al. [11] reported that even variations in morphine dose could explain less than half of the variability of blood levels of morphine metabolites in patients with cancer. Thus, using an external criterion may increase uncertainty about an individual’s group membership, and this uncertainty cannot be quantified in the form of probabilities.
The group-based trajectory model (GBTM) is a statistical approach designed to group longitudinal observations into interrelated subgroups. Like cross-sectional methods, GBTM takes into consideration measures at a given time point, but unlike other methods, GBTM considers the change patterns of those measures across multiple time points. It is able to identify distinctive developmental trajectories and to provide, for model evaluation, the probability of population members’ following each such trajectory [12]. GBTM outputs are easily understood graphs and tables of longitudinal measurements, which may be more accessible for both clinicians and researchers.
Because GBTM was originally developed to describe the course of behavioral outcomes related to age [13], it has primarily been used to establish developmental trajectories in psychology or criminology in populations of children or adolescents. Researchers have used GBTM to analyze outcomes such as physical aggression [14], depression and anxiety symptoms [15], and adherence to treatment [16]. GBTM’s applicability in cancer symptom research is not well established. In a study of symptoms in lung cancer patients undergoing chemotherapy [17], we used GBTM to identify two subgroups, a high-symptom group and a low-symptom group. In that study, the two-group model was determined a priori for simplicity and clinical usefulness. How this two-group model is different from a model selected by a statistical-fit index, such as the Bayesian information criterion (BIC), has not been addressed. Moreover, GBTM’s ability to detect predictors for higher symptom burden should be compared with that of the commonly used LMM.
On the basis of previous cross-sectional research [2], for this longitudinal study we hypothesized that symptom burden would be heterogeneous in patients with HNC undergoing radiotherapy or chemoradiotherapy, and that GBTM would identify a subgroup of patients with high symptom burden. In addition, we compared the capabilities of a predetermined two-group GBTM model, a statistical best-fit GBTM model, and an LMM model for identifying predictors of high symptom burden.
Methods
Patients
Patients with HNC who were qualified to receive radiotherapy or chemoradiotherapy were recruited from the Head and Neck Planning and Development Clinic [18] at The University of Texas MD Anderson Cancer Center between February 2006 and August 2007. All patients were 18 year of age or older. The study was approved by the MD Anderson Institutional Review Board, and all patients gave written informed consent to participate prior to the baseline evaluation. We analyzed data from non-Hispanic white patients only, because very few other ethnic groups were represented in this cohort.
Symptom measurements
The M. D. Anderson Symptom Inventory (MDASI) is a psychometrically validated and widely used instrument for cancer symptom measurement [19]; the MDASI Head and Neck module (MDASI-HN) has been validated for use in this patient population [20]. The 28-item MDASI-HN comprises three subscales: 13 core MDASI items that rate the severity of general symptoms associated with cancer, six interference items that assess how severely symptoms interfere with daily activities, and nine HNC-specific items that rate the severity of symptoms particularly associated with HNC. The core and HNC-specific symptoms are rated on a 0–10 scale to indicate the presence and severity of the symptom, with 0 indicating “not present” and 10 indicating “as bad as you can imagine.” Patients are asked to rate each item according to its worst severity during the previous 24 hours.
Patients completed MDASI-HN assessments once a week for 10 weeks beginning at the start of radiotherapy or chemoradiotherapy.
Statistical analysis
An average score of the top five symptoms, determined as the five most severe symptoms at the end of treatment (week 7), was calculated. With this average score as the dependent variable, GBTM was used to identify patient subgroups with distinct symptom-development trajectories over the course of therapy. SAS macro PROC TRAJ [21] was used to estimate the trajectories, on the basis of data collected at 11 time points (from before treatment to week 10). First, we generated a two-group model with the prior of simplicity and clinical interpretability, representing either high or low symptom burden over time of the 10-week study. Next, a second model with the optimal number of groups as determined by the lowest BIC was generated. Mplus was used to conduct bootstrap likelihood ratio tests (BLRT) [6] and Lo-Mendell-Rubin adjusted likelihood ratio tests (LMR LRT) [22] as additional tests to confirm the optimal number of groups.
We then used three diagnostics to evaluate the adequacy of the selected models [12]: (a) the average of the posterior probabilities of group membership for individuals assigned to each group exceeded 0.7, (b) the odds of correct classification exceeded 5, and (c) the estimated probability of group membership differed by less than 50% from the proportion assigned to that group on the basis of the posterior probability of group membership. Descriptive statistics, including means, standard deviations (SD), percentages, odds ratios (OR), and 95% confidence intervals (95% CI), are used to report patient demographic and clinical characteristics by group for the two models.
To identify predictors of high symptom burden, we included potential predictors in the TRAJ models. Risk variables included age (≥60 years vs. <60 years), sex (male vs. female), Eastern Cooperative Oncology Group performance status (ECOG PS) score (≥1 vs. 0), treatment (chemoradiotherapy vs. radiotherapy), cancer stage (III/IV vs. other), cancer diagnosis (pharynx vs. other), and total radiation dose (>60 Gy vs. ≤60 Gy). To control the possible association between early dropout from the study and symptom severity, we added a variable indicating early dropout status (dropout before week 10 vs. completing the investigation) in all models.
We then compared ability to describe longitudinal symptom burden between GBTM and LMM, the most commonly used approach. Considering the component score for the top five symptoms as a continuous variable, we used LMM to estimate the associations between overall symptom development and factors involved in the previous logistic regression model.
SAS 9.3 and Mplus 6.11 were used to conduct all analyses. All statistical tests were two-sided; P values < 0.05 were considered statistically significant.
Results
Demographic and clinical characteristics
One hundred thirty patients were enrolled in this study. At the end of investigation (week 10), 82 patients (63%) remained in the cohort. Patient characteristics are shown in Table 1. No significant differences in demographic and clinical factors were found between those who dropped out before week 10 and those who did not (Table 1). The top five symptoms at week 7 were problems with tasting food (mean 6.28, SD 3.72), difficulty chewing or swallowing (mean 5.23, SD 3.65), mucus (mean 5.77, SD 3.50), fatigue (mean 5.27, SD 2.79), and dry mouth (mean 5.67, SD 3.51).
Table 1.
Patient characteristics at baseline (pretreatment)
| Total (N = 130) | Investigation completed (n = 82) |
Early dropout (n = 48) |
P* | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age | .579 | ||||||
| < 60 years | 61 | 46.92 | 40 | 48.78 | 21 | 43.75 | |
| ≥ 60 years | 69 | 53.08 | 42 | 51.22 | 27 | 56.25 | |
| Sex | .617 | ||||||
| Women | 32 | 24.62 | 19 | 23.17 | 13 | 27.08 | |
| Men | 98 | 75.38 | 63 | 76.83 | 35 | 72.92 | |
| Tumor stage | .457 | ||||||
| 0/I/II | 84 | 64.62 | 55 | 67.07 | 29 | 60.42 | |
| III/IV | 36 | 27.69 | 21 | 25.61 | 15 | 31.25 | |
| ECOG PS | .169 | ||||||
| 0 | 88 | 67.69 | 59 | 71.95 | 29 | 60.42 | |
| ≥ 1 | 37 | 28.46 | 20 | 24.39 | 17 | 35.42 | |
| Treatment | .323 | ||||||
| Radiotherapy | 52 | 40.00 | 35 | 42.68 | 17 | 35.42 | |
| Chemoradiotherapy | 75 | 57.69 | 44 | 53.66 | 31 | 64.58 | |
| Total radiation dose | .853 | ||||||
| ≤ 60 Gy | 81 | 62.31 | 51 | 62.20 | 30 | 62.50 | |
| > 60 Gy | 48 | 36.92 | 31 | 37.80 | 17 | 35.42 | |
| Cancer site | .504 | ||||||
| Pharynx | 62 | 47.69 | 42 | 51.22 | 20 | 41.67 | |
| Tongue | 23 | 17.69 | 15 | 18.29 | 8 | 16.67 | |
| Oral | 10 | 7.69 | 4 | 4.88 | 6 | 12.50 | |
| Larynx | 10 | 7.69 | 6 | 7.32 | 4 | 8.33 | |
| Paranasal sinus | 10 | 7.69 | 7 | 8.54 | 3 | 6.25 | |
| Salivary | 6 | 4.62 | 3 | 3.66 | 3 | 6.25 | |
| Thyroid | 4 | 3.08 | 1 | 1.22 | 3 | 6.25 | |
| Skin | 4 | 3.08 | 3 | 3.66 | 1 | 2.08 | |
Chi square test comparing number of patients who did or did not drop out before week 10.
GBTM selection and evaluation
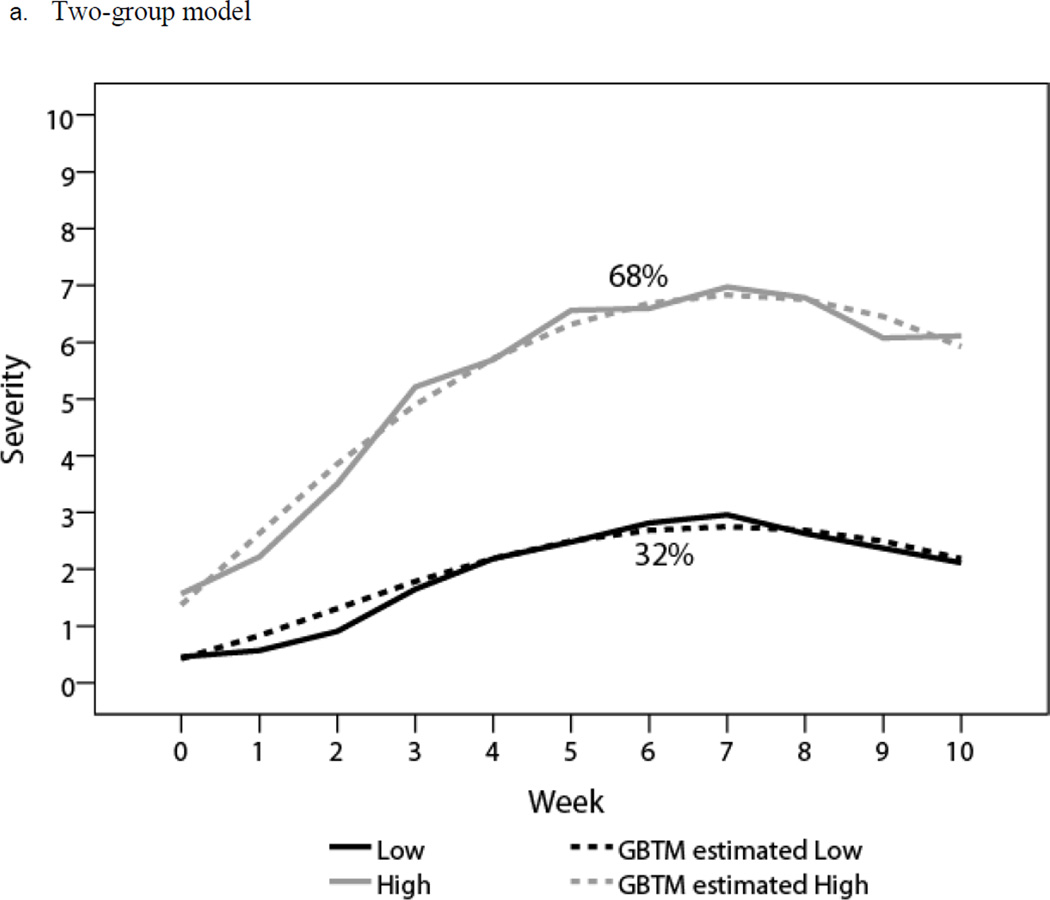
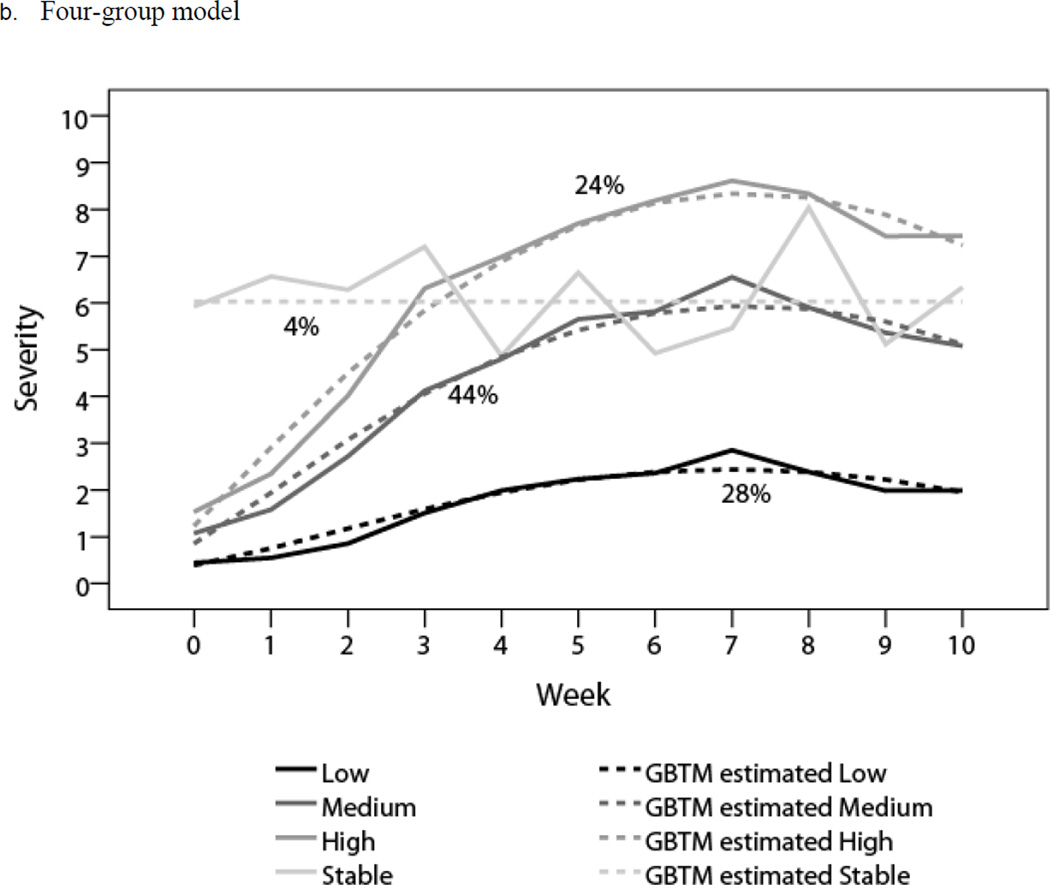
GBTM results indicated that a four-group solution had the smallest BIC; BLRT showed that a four-group model was superior to one-, two-, and three-group models, whereas LMR LRT showed that a two-group model was optimal (Table 2). Accordingly, we generated both two-group and four-group models. Both models met the criteria for adequate model fit (Table 3). We defined the two-group symptom trajectories as low (32% of patients) and high (68%), and the four-group symptom trajectories as low (28% of patients), medium (44%), high (24%), and stable (4%).
Table 2.
Identification of optimal group number
| 2-group model | 3-group model | 4-group model | 5-group model | ||
|---|---|---|---|---|---|
| BIC* | −2188.33 | −2147.10 | −2129.47 | −2133.02 | |
| BLRT* n- vs. (n−1)-group model | |||||
| 2×Loglikelihood difference | 325.892 | 97.633 | 36.045 | 4.529 | |
| Approximate P-Value | 0.000 | 0.0000 | 0.0000 | 0.339 | |
| LO-MENDELL-RUBIN adjusted LRT* test n- vs. (n−1)-group model | |||||
| Value | 309.972 | 92.863 | 43.082 | 34.284 | |
| P | 0.0000 | 0.0579 | 0.2265 | 0.6177 | |
BIC: Bayesian Information criterion; BLRT: bootstrapped parametric likelihood ratio test; LRT: likelihood ratio test
Table 3.
Group-based trajectory model diagnostics
| Model | AvePP | OCCj | Pj | πj | % difference between Pj and πj |
|---|---|---|---|---|---|
| Two-group | |||||
| Low | 0.96 | 51.00 | 0.329 | 0.32 | 2.81 |
| High | 0.97 | 15.68 | 0.671 | 0.68 | 1.32 |
| Four-group | |||||
| Low | 0.95 | 48.86 | 0.281 | 0.28 | 0.36 |
| Medium | 0.91 | 12.86 | 0.434 | 0.44 | 1.36 |
| High | 0.90 | 28.50 | 0.244 | 0.24 | 1.67 |
| Stable | 0.99 | 2376.00 | 0.041 | 0.04 | 2.50 |
AvePP: average of the posterior probabilities of group membership; OCCj: odds of correct classification; Pj: posterior probabilities of group membership; πj: proportion of group membership.
The profiles of these two models are shown in Table 4. Estimated means (dashed lines) and observed means (solid lines) at each time point for all subgroups are plotted in Figure 1. In the two-group model, both groups demonstrated significant linear and quadratic terms, with a mild symptom peak (mean 2.96, SD 1.58) for the low group and a moderate symptom peak (mean 6.98, SD 1.90) for the high group (Figure 1a). In the four-group model, the low, medium, and high trajectories demonstrated significant linear and quadratic terms, starting with low symptom burden, increasing to a peak at the end of treatment (week 7), and then decreasing. The peak levels of symptoms were mild for the low group (mean 2.80, SD 1.59), moderate for the medium group (mean 6.56, SD 1.97), and severe for the high group (mean 8.65, SD 0.91). The stable group started with a moderate symptom burden (mean 6.04, SD 2.93) that remained level throughout the investigation (Figure 1b).
Table 4.
Group-based trajectory model profiles
| Model parameters, estimate (P) | Severity of top five symptoms, mean (SD) | |||||
|---|---|---|---|---|---|---|
| Intercept | Linear | Quadratic | Baseline | Week 7 | Week 10 | |
| Two-group model | ||||||
| Low (n = 42) | −0.65 (< .010) | 0.96 (< .0001) | −0.07 (< .0001) | 0.43 (0.64) | 2.96 (2.05) | 2.05 (1.38) |
| High (n = 88) | 1.08 (< .0001) | 1.61 (< .0001) | −0.11 (< .0001) | 1.57 (1.80) | 6.98 (1.90) | 6.12 (1.82) |
| Four-group model | ||||||
| Low (n = 37) | −0.51 (.0295) | 0.83 (< .0001) | −0.06 (< .0001) | 0.43 (0.67) | 2.80 (1.59) | 1.99 (1.41) |
| Medium (n = 57) | 0.44 (.016) | 1.52 (< .0001) | −0.11 (< .0001) | 1.10 (1.22) | 6.56 (1.97) | 5.07 (1.57) |
| High (n = 31) | 0.99 (.0001) | 2.04 (< .0001) | −0.14 (< .0001) | 1.53 (1.41) | 8.65 (0.91) | 7.50 (1.50) |
| Stable (n = 5) | 6.03 (< .0001) | - | - | 6.04 (2.93) | 5.45 (0.07) | 6.33 (0.76) |
Fig. 1.
Trajectories for the five most-severe symptoms in patients with head and neck cancer
Patient characteristics by group
Tables 5 and 6 demonstrate the distribution of patient demographic and clinical characteristics by group. Significantly higher proportions of patients who were receiving chemoradiotherapy, were 60 years of age or older, had stage III/IV cancer, and had ECOG PS ≥ 1 were found in the high-symptom subgroup of the two-group model and in the medium-symptom subgroup of the four-group model than were found in the low-symptom subgroups of either model. No comparison was done with the stable group because of its small sample size (n = 5).
Table 5.
Patient characteristics of the two-group model
| Symptom burden | |||||
|---|---|---|---|---|---|
| Group | Low (n = 42) | High (n = 88) | P* | ||
| n | % | n | % | ||
| Treatment | |||||
| Radiotherapy** | 23 | 56.10 | 29 | 33.72 | .017 |
| Chemoradiotherapy** | 18 | 43.90 | 57 | 66.28 | |
| Age | |||||
| < 60 years | 26 | 61.90 | 35 | 39.77 | .018 |
| ≥ 60 years | 16 | 38.10 | 53 | 60.23 | |
| Sex | |||||
| Women | 13 | 30.95 | 19 | 21.59 | .247 |
| Men | 29 | 69.05 | 69 | 78.41 | |
| Tumor stage | |||||
| 0/I/II | 32 | 86.49 | 52 | 62.65 | .009 |
| III/IV | 5 | 13.51 | 31 | 37.35 | |
| Cancer site | |||||
| Pharynx | 20 | 48.78 | 42 | 47.73 | .894 |
| Tongue | 1 | 2.44 | 22 | 25.00 | .002 |
| ECOG PS | |||||
| 0 | 36 | 92.31 | 52 | 60.47 | .0003 |
| ≥ 1 | 3 | 7.69 | 34 | 39.53 | |
| Total radiation dose | |||||
| ≤ 60 Gy | 30 | 71.43 | 51 | 58.62 | .159 |
| > 60 Gy | 12 | 28.57 | 36 | 41.38 | |
Chi square test
Table 6.
Patient characteristics of the four-group model
| Symptom burden |
P
* (medium vs. low) |
P
* (high vs. low) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low (n = 37) |
Medium (n = 57) |
High (n = 31) |
Stable (n = 5) |
|||||||
| n | % | n | % | n | % | n | % | |||
| Treatment | ||||||||||
| Radiotherapy | 21 | 58.33 | 19 | 34.55 | 12 | 38.71 | . | . | .025 | .109 |
| Chemoradiotherapy | 15 | 41.67 | 36 | 65.45 | 19 | 61.29 | 5 | 100.00 | ||
| Age | ||||||||||
| < 60 years | 24 | 64.86 | 21 | 36.84 | 15 | 48.39 | 1 | 20.00 | .008 | .171 |
| ≥ 60 years | 13 | 35.14 | 36 | 63.16 | 16 | 51.61 | 4 | 80.00 | ||
| Sex | ||||||||||
| Women | 13 | 35.14 | 9 | 15.79 | 10 | 32.26 | . | . | .030 | .803 |
| Men | 24 | 64.86 | 48 | 84.21 | 21 | 67.74 | 5 | 100.00 | ||
| Tumor stage | ||||||||||
| 0/I/II | 29 | 87.88 | 34 | 66.67 | 19 | 61.29 | 2 | 40.00 | .028 | .014 |
| III/IV | 4 | 12.12 | 17 | 33.33 | 12 | 38.71 | 3 | 60.00 | ||
| Cancer site | ||||||||||
| Pharynx | 18 | 50.00 | 23 | 40.35 | 16 | 51.61 | 5 | 100.00 | .533 | .807 |
| Tongue | 1 | 2.78 | 16 | 28.07 | 6 | 19.35 | 0 | 0.00 | .002 | .024 |
| ECOG PS | ||||||||||
| 0 | 32 | 94.12 | 39 | 69.64 | 15 | 50.00 | 2 | 40.00 | .006 | < .0001 |
| ≥ 1 | 2 | 5.88 | 17 | 30.36 | 15 | 50.00 | 3 | 60.00 | ||
| Total radiation dose | ||||||||||
| ≤ 60 Gy | 25 | 67.57 | 37 | 66.07 | 16 | 51.61 | 3 | 60.00 | .881 | .181 |
| > 60 Gy | 12 | 32.43 | 19 | 33.93 | 15 | 48.39 | 2 | 40.00 | ||
Chi square test
Predictors of high symptom burden
Because of the small sample size of patients with tongue cancer in some subgroups, we did not include tongue cancer in the multivariate logistic models. For two-group model, the predictors of being assigned to the high-symptom group included having an ECOG PS ≥ 1 (est = 2.97; P =0.002) and receiving chemoradiotherapy (est=1.23; P = 0.046). For the four-group model, predictors for being assigned to the medium group included being female (est = 2.06; P = 0.022), being at least 60 years old (est = 1.27; P = .048), and receiving chemoradiotherapy (est = 1.64; P = 0.027); the predictor for being assigned to the high group was having an ECOG PS ≥ 1 (est = 2.73; P = 0.003); and the predictor of being assigned to the stable group was having an ECOG PS ≥ 1 (est = 3.89; P = 0.007) (Table 7).
Table 7.
Predictors of high symptom burden
| Parameter | Estimate | SE | P |
|---|---|---|---|
| Two-group model (high vs. low)* | |||
| ECOG PS ≥ 1 | 2.97 | 0.96 | 0.002 |
| Concurrent chemoradiotherapy | 1.23 | 0.62 | 0.046 |
| Four-group model (medium vs. low)* | |||
| Sex (female) | 2.06 | 0.89 | .022 |
| Age ≥60 years | 1.27 | 0.64 | .048 |
| Concurrent chemoradiotherapy | 1.64 | 0.74 | .027 |
| Four-group model (high vs. low)* | |||
| ECOG PS ≥ 1 | 2.73 | 0.92 | .003 |
| Four-group model (stable vs. low) | |||
| ECOG PS ≥ 1 | 3.89 | 1.43 | 0.007 |
| Linear mixed-effect model* | |||
| Week | 1.33 | 0.06 | <.0001 |
| Week*week | −0.09 | 0.01 | <.0001 |
| Age ≥ 60 years | 0.61 | 0.30 | 0.045 |
| ECOG PS ≥ 1 | 1.37 | 0.33 | <.0001 |
Variables included in models: Age, sex, cancer site, tumor stage, total radiation does radiotherapy, ECOG PS, treatment methods, and early drop-out.
With the component score for the top five symptoms from before treatment to week 10 as a continuous dependent variable, the LMM generated significant linear (est = 1.33; P < .0001) and quadratic (est = −0.09; P < .0001) terms over time. The model identified ECOG PS ≥ 1 (est = 1.37; P < .0001) and age 60 years or older (est = 0.61; P = .045) as predictors of higher symptom burden (Table 7).
Discussion
Our results confirmed the hypothesis that GBTM can identify subgroups of patients with HNC who experience high symptom burden during chemoradiotherapy or radiotherapy. The model fit of the two-group GBTM was as good as that of the four-group GBTM selected according to BIC. In our sample, 67% of patients in the two-group GBTM had high symptom burden over time, represented by the five most-severe symptoms. The four-group model identified a subgroup of patients (4%) who started with moderate to high symptom burden that remained stable over the course of therapy, whereas the two-group model did not differentiate those patients from those in the high symptom burden group. All three models (the two-group GBTM, four-group GBTM, and LMM) exhibited significant linear and quadratic functions for symptom change over the course of chemoradiotherapy or radiotherapy. Using the GBTM group membership, we identified age 60 years or older, poor baseline performance status (ECOG PS ≥ 1), and receiving chemoradiotherapy (vs. radiation alone) as predictors of higher symptom burden. The LMM identified two predictors: ECOG PS ≥ 1 and age 60 years or older.
The consistency in symptom development across models suggests that most patients’ symptom development patterns were homogeneous, increasing to the end of therapy and then dropping, regardless of symptom severity. However, the increasing trend estimated by LMM was lower than that in the medium and high subgroups obtained from GBTM. Because LMM is based on the assumption that all patients’ patterns of change are homogeneous, it may underestimate the symptom change for some patients and may overestimate the change for others. In contrast, the GBTM tested heterogeneity in symptom change and identified patient subgroups characterized by varied symptom severity levels.
The two-group GBTM demonstrated that, although all patients started with low symptom burden, two-thirds reported significantly more-severe symptoms during treatment. This result is consistent with results from a cluster analysis conducted at the end of treatment in a patient set partly overlapping with our population [2]; in that analysis, 59% of patients were in the high-symptom group at week 6.
The four-group model generated a similar proportion of patients belonging to subgroups with moderate or high symptom burden. Notably, the four-group model also identified a stable subgroup (4%) who started with moderate to high symptoms and retained that level of symptom burden throughout the course of investigation, without the initial rising and subsequent falling pattern found in other subgroups. A subgroup of patients (30%) with stably high symptoms driven by cancer stage was found in our previous study of patients undergoing chemotherapy for advanced lung cancer [17]. Although the small size of the stable group (only five patients) in the current study hindered further analysis, these results suggests that the four-group GBTM may be more sensitive than the other two models in describing interindividual heterogeneity.
Poor performance status (ECOG PS) at baseline was identified by both GBTM and LMM models as a predictor for high symptom burden. As the most-used measurement of performance status, ECOG PS is highly correlated with symptom scores and has been used for symptom-instrument validation as the gold standard for assessing patients’ general condition [19]. The consistent results suggest that the GBTM trajectories were as capable as LMM of identifying symptom variations related to varied performance status. However, the interpretation of the results is different, both clinically and statistically. The LMM described patients with ECOG PS ≥ 1 as being more likely to report greater symptom burden over time, whereas GBTM described those patients as being more likely to follow the patterns demonstrated in medium and high symptom subgroups.
It is widely accepted that the toxicities of aggressive cancer therapy can result in moderate to severe symptom levels during the course of treatment [8, 23]. Although concurrent chemoradiotherapy has been reported to yield a significant survival benefit [24], Rosenthal et al. [23] reported that chemoradiotherapy led to more-severe and longer-lasting mucositis compared with radiotherapy alone. In our sample, treatment with chemoradiotherapy was associated with a higher probability of developing moderate or high symptom burden compared with radiotherapy alone. However, the LMM did not reveal an association between symptom severity and treatment methods; possibly, the mixing of patients experiencing low symptom burden with those experiencing moderate and high symptom burden may have weakened the association between treatment and symptoms, considering that patients who maintain mild symptom levels over time may not be responsive to the toxicities of either chemoradiotherapy or radiotherapy. In such situations, GBTM is more efficient for capturing individual difference of response to therapy, thus providing a better method for identifying patients at higher risk for developing severe symptoms.
Our moderate sample size imposed limitations in GBTM application. One concern with a small sample is GBTM’s difficulty in detecting subgroups that represent a very small portion of the population. The small subgroup of patients who maintained moderate or high symptom burden throughout therapy was not identified by the two-group model. In a larger sample of more-symptomatic patients with lung cancer [17], we identified such a subgroup using a similar two-group model. In addition, the small sample size of each subgroup in the current study limited the further application of GBTM trajectories.
For any analytical method, a small sample may result in higher likelihood that the results were based on chance variation. In our analysis of the four-group model, the wide 95% CI ranges of ORs for predictors of high symptom burden bring uncertainty to the interpretation of those associations. Although the two-group model failed to identify the stable subgroup, it did identify the same predictor panel as the four-group model did, but with narrower 95% CI ranges. Thus, when the GBTM is applied to a small or moderate-size sample, statistical indices should not be the only criterion for model selection. The interpretability and clinical usefulness of reporting distinctive developmental patterns in the data should also be considered.
The other limitation of our study was that a substantial amount of data (37%) was missing from the 10-week-long investigation. Because the GBTM uses all available data, data missing due to symptom worsening may result in misclassification. In the current analysis, we added the dropout status as a covariate in all models and found no differences between those who dropped out and those who did not. Similarly, the LMM did not identify a significant effect of early dropout on overall symptom development. The consistent results indicate that early dropout in this population was not related to symptom severity and may not affect the modeling based on all available symptom severity data.
Another limitation of the GBTM procedure itself is that it may simply be identifying extra groups to accommodate non-normality in the data, rather than true latent subgroups [25, 26]. Residual diagnostics indicated that the two-group model, but not the three- or four-group models, is free of extra groups due to non-normality. This suggests that the two-group model reflects the heterogeneity of our symptom data, whereas there is a possibility that the three- or four-group models were identifying a mixture distribution when in fact it was a homogeneous non-normal density [27, 28]. Finally, we compared risk factor results using the conventional one-group model, the LMM, and two- and four-group models. Being treated with concurrent chemoradiation therapy was identified as predictor by multi-group models, but not by the onegroup model. Meanwhile, in the four-group model, predictors varied across groups: the medium group was associated with sex and treatment type, while the other two groups (high and stable) were related to ECOG PS (Table 7). Those variations suggest that compared with the one-group model, multi-group models make meaningful distinctions among patients. In clinical practice, the presence of multiple groups will help to isolate the subgroup of patients who may need specific medical attention when they are undergoing concurrent chemoradiation therapy.
In conclusion, GBTM can identify distinct developmental trajectories of symptoms in patients with HNC and should be applicable for the examination of the symptom trajectories of other treatments (both cancer and noncancer) known to increase symptoms. The heterogeneities described by GBTM can be partly attributed to several patient characteristics. The inconsistency between GBTM and LMM results highlights the importance of selecting the appropriate method on the basis of research questions and the nature of the target population. Because of the interindividual variation of response to aggressive cancer therapy, GBTM is useful for describing the heterogeneity of symptom development, for identifying groups to determine demographic or biological risk factors, and potentially for informing clinicians about subgroups of patients who will need more attention to symptom control.
Acknowledgments
The project described was supported by awards from the National Institutes of Health: Award Number CA016672, a Cancer Center Support Grant to The University of Texas MD Anderson Cancer Center, and Award Number CA026582 to Charles S. Cleeland, PhD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
The authors acknowledge the editorial assistance of Jeanie F. Woodruff, BS, ELS.
References
- 1.Haigentz M, Jr, Corry J, Strojan P, Ferlito A. Easing acceleration of head and neck chemoradiotherapy. Lancet Oncol. 2012;13(2):113–115. doi: 10.1016/S1470-2045(11)70382-5. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal DI, Mendoza T, Cleeland C. Identifying head and neck patients at risk for high symptom burden during treatment [abstract]. American Society of Clinical Oncology 2009 Annual Meeting, Orlando FL, May 29 – Jun 2, 2009. J Clin Oncol. 2009;27(15 Suppl) Abstract #6066. [Google Scholar]
- 3.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur. J. Oncol. Nurs. 2010;14(2):101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and qualityof- life outcomes: a cluster analysis. Oncol. Nurs. Forum. 2006;33(5):E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 5.Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Studies of Cancer Survivors. Cancer. 2011;117(12):2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78(6):1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 7.Dodd MJ, Cho MH, Cooper BA, Petersen J, Bank KA, Lee KA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol. Nurs. Forum. 2011;38(1):33–42. doi: 10.1188/11.ONF.33-42. [DOI] [PubMed] [Google Scholar]
- 8.Wang XS, Fairclough DL, Liao Z, Komaki R, Chang JY, Mobley GM, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24(27):4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 9.Langford DJ, Tripathy D, Paul SM, West C, Dodd MJ, Schumacher K, et al. Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain. J. Pain. 2011;12(4):495–507. doi: 10.1016/j.jpain.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merriman JD, Jansen C, Koetters T, West C, Dodd M, Lee K, et al. Predictors of the trajectories of self-reported attentional fatigue in women with breast cancer undergoing radiation therapy. Oncol Nurs Forum. 2010;37(4):423–432. doi: 10.1188/10.ONF.423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepstad P, Dale O, Kaasa S, Zahlsen K, Aamo T, Fayers P, et al. Influences on serum concentrations of morphine, M6G and M3G during routine clinical drug monitoring: a prospective survey in 300 adult cancer patients. Acta Anaesthesiol. Scand. 2003;47(6):725–731. doi: 10.1034/j.1399-6576.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 13.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Brame B, Nagin DS, Tremblay RE. Developmental trajectories of physical aggression from school entry to late adolescence. J Child Psychol Psychiatry. 2001;42(4):503–512. [PubMed] [Google Scholar]
- 15.Côté SM, Boivin M, Liu X, Nagin DS, Zoccolillo M, Tremblay RE. Depression and anxiety symptoms: onset, developmental course and risk factors during early childhood. J Child Psychol Psychiatry. 2009;50(10):1201–1208. doi: 10.1111/j.1469-7610.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 16.Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of Adherence to Airway Clearance Therapy for Patients with Cystic Fibrosis. J Pediatr Psychol. 2010 doi: 10.1093/jpepsy/jsq015. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Mendoza TR, Wang XS, Woodruff JF, Palos GR, Richman SP, et al. Levels of symptom burden during chemotherapy for advanced lung cancer: differences between public hospitals and a tertiary cancer center. J. Clin. Oncol. 2011;29(21):2859–2865. doi: 10.1200/JCO.2010.33.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal DI, Asper JA, Barker JL, Jr, Garden AS, Chao KS, Morrison WH, et al. Importance of patient examination to clinical quality assurance in head and neck radiation oncology. Head Neck. 2006;28(11):967–973. doi: 10.1002/hed.20446. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal DI, Mendoza TR, Chambers MS, Asper JA, Gning I, Kies MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M.D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 21.Jones BL. [Accessed 22 May 2012];TRAJ: group-based modeling of longitudinal data. 2005 http://www.andrew.cmu.edu/user/bjones/
- 22.Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 23.Rosenthal DI, Mendoza TR, Chambers MS, Burkett VS, Garden AS, Hessell AC, et al. The M. D. Anderson symptom inventory-head and neck module, a patient-reported outcome instrument, accurately predicts the severity of radiation-induced mucositis. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(5):1355–1361. doi: 10.1016/j.ijrobp.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 24.Budach W, Hehr T, Budach V, Belka C, Dietz K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC. Cancer. 2006;6:28. doi: 10.1186/1471-2407-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthén B. Statistical and substantive checking in growth mixture modeling: Comment on Bauer and Curran (2003) Psychological Methods. 2003;8(3):369–377. doi: 10.1037/1082-989X.8.3.369. [DOI] [PubMed] [Google Scholar]
- 26.Muthén B. Latent variable analysis: growth mixture modeling and related techeniques for longitudinal data. In: Kaplan D, editor. The SAGE Handbook of Quantitative Methodology for the Social Sciences. Thousand Oaks, CA: Sage Publications, Inc.; 2004. pp. 345–368. [Google Scholar]
- 27.Tarpey T, Yun D, Petkova E. Model Misspecification: Finite Mixture or Homogeneous? Stat. Modelling. 2008;8(2):199–218. doi: 10.1177/1471082X0800800204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: Implications for overextraction of latent trajectory classes. Psychological Methods. 2003;8(3):338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]