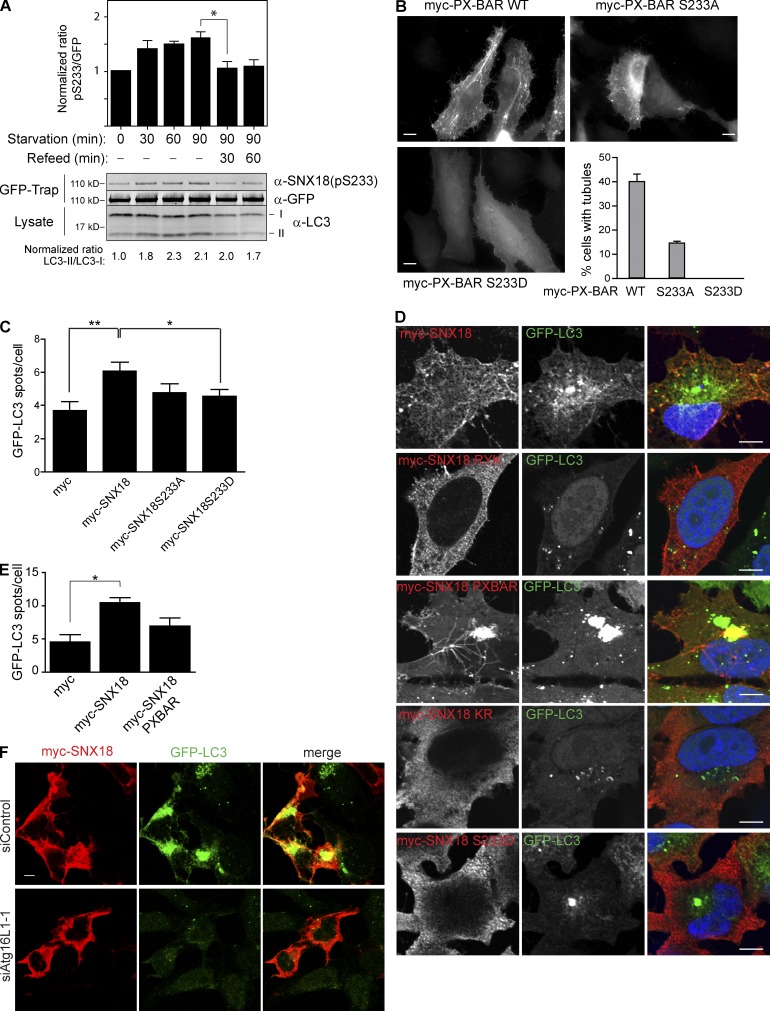
Figure 5.
The tubulation activity of SNX18 is important for its role in autophagy and is regulated by phosphorylation. (A) HeLa GFP-SNX18 cells were starved for the indicated time, followed by incubation in full medium (Refeed) where indicated. Cell lysates were analyzed for LC3 by immunoblotting, or immunoprecipitated with GFP-Trap before immunoblotting against SNX18(pS233) and GFP. The ratio of pS233 to GFP, and the ratio of LC3-II to LC3-I, was quantified, and normalized to 1 for the zero-time sample in both cases (graph shows mean ± SEM [error bars], n = 4; *, P < 0.05). (B) The indicated myc-SNX18 PX-BAR constructs were transfected into HeLa cells, and their tubulation efficiency was measured by scoring the percentage of cells displaying more than three elongated structures longer than 5 µm (n = 300). The graph shows the mean of two independent experiments ± range (error bars). Bars, 10 µm. (C) The indicated myc-tagged SNX18 constructs were transfected into HEK GFP-LC3 cells, and the number of GFP-LC3 spots per cell was quantified. The graph shows mean ± SEM (error bars), n = 5. (D) HeLa GFP-LC3 cells were transfected with the indicated myc-tagged SNX18 constructs for 16 h, starved for 2 h, and analyzed by confocal imaging. Bars, 10 µm. (E) HEK GFP-LC3 cells were transfected with myc-SNX18 WT or myc-SNX18 PX-BAR constructs, and the number of GFP-LC3 spots per cell was quantified. The graph shows mean ± SEM (error bars), n = 3. (F) HeLa GFP-LC3 cells were transfected with siRNA against Atg16L1 and later with myc-SNX18 constructs before immunostaining and confocal imaging. Bar, 5 µm.