Figure 1.
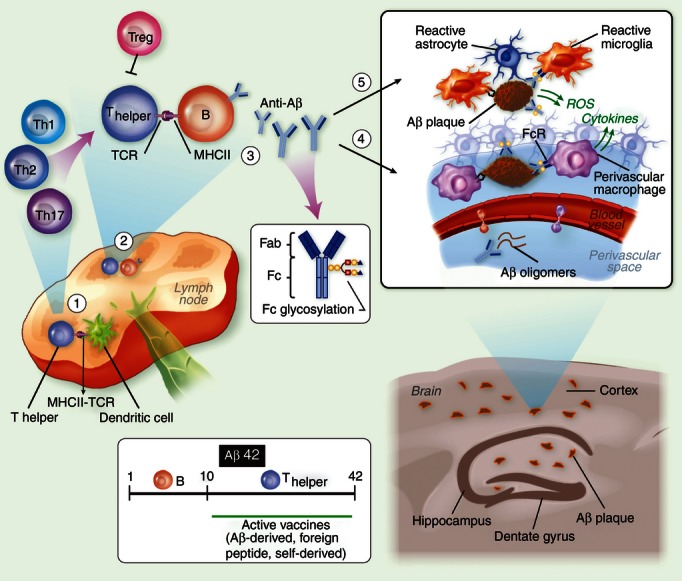
Amyloid β antibody-based immunotherapy of Alzheimer's disease (AD). (1) The immune response elicited by Aβ immunization begins in draining lymph nodes where dendritic cells present the MHC II-bound antigen to naive CD4 T cells. The antigen may be Aβ42 or a shorter peptide conjugated to a carrier of choice, such as heat-shock protein p458 or PADRE. (2) T cells then migrate to B-cell follicles, where they promote Aβ-specific B-cell proliferation and differentiation to plasma and memory B cells. Depending on the adjuvant and the carrier used, T cells polarize to either a pro-inflammatory or anti-inflammatory phenotype. In the case of Aβ or other self-derived carriers, T-cell responsiveness may be attenuated because of anergy or the presence of specific regulatory T cells. (3) Secreted antibodies may be of various isotypes and specificities to Aβ42 and with various glycoforms at the Fc portion, processes regulated by the cytokine milieu. (4) Antibodies target Aβ at the brain vasculature and enhance Aβ clearance from the brain. Clearance of soluble Aβ is accomplished through perivascular drainage or Fc receptor (FcR) -mediated uptake. Clearance of compact Aβ plaques is less effective, although the activation of perivascular macrophages via activating FcR may enhance their phagocytic function. Such a reaction, however, can facilitate an inflammatory reaction at the brain vasculature influenced by microglial/macrophage scavenger receptors114,115 and also by the Aβ B-cell epitope, the Fc glycosylation pattern and/or the type of Fc receptors (i.e. activating or inhibitory FcRs). The inflammatory reaction at the vasculature may also be influenced by the adjuvant and carrier of choice, a process that may enhance clearance on the one hand but promote brain inflammation and microhaemorrhages on the other. (5) Similar processes occur within the brain parenchyma once antibodies target Aβ plaques. As some monocytes infiltrate the brain and target Aβ plaques, the capacity of antibody-mediated clearance may increase.
