Figure 2.
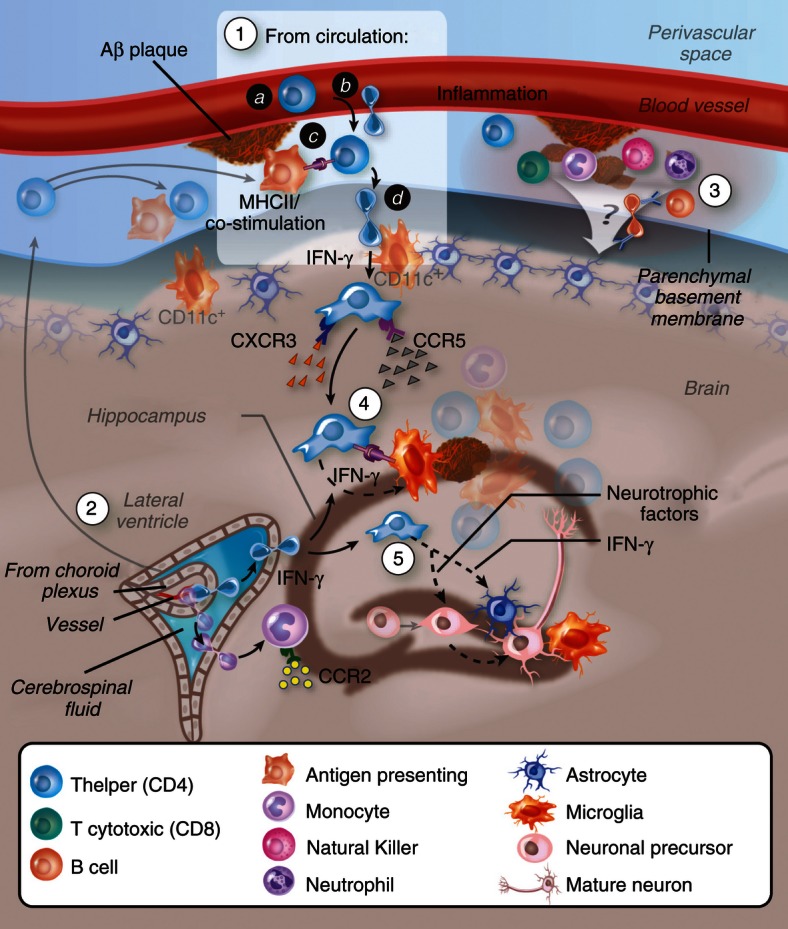
Migration of Aβ-specific T cells towards Aβ plaques in the brain parenchyma. (1) T cells may undergo activation following Aβ immunization or following drainage of Aβ or antigen-presenting cells (APCs) that carry Aβ to peripheral lymph nodes. Aβ-reactive helper T (Th) cells adhere and transmigrate into the perivascular space of Aβ-deposited vasculature in the brain (a, b). To cross the glia limitans, Th cells need to be re-stimulated by dendritic cells or, possibly, by other competent APCs located at the perivascular space and/or juxtavascular with processes sent into the perivascular space.116 Similar T-cell infiltration processes may occur at the choroid plexus (2), and/or the leptomeninges85,117,118 followed by their dissemination in the central nervous system subarachnoid space. Low levels of interferon-γ (IFN-γ) promote the infiltration process. Adhesion molecules such as P-selectin, vascular cell adhesion molecule-1 or intercellular adhesion molecule 1 (interacting with P-selectin glycoprotein ligand-1, integrin α4 and lymphocyte function-associated antigen-1, respectively, on the T cells) and chemokine signalling (such as via CCR5 and CXCR3) play a key role in mediating the extravasation of the T cells through the blood–brain barrier (BBB) or the blood–cerebrospinal fluid barrier. (3) Leucocytes accumulate at the subarachnoid and perivascular spaces and may impact on the overall inflammatory reaction at both the vasculature and parenchyma. (4) Once Aβ Th cells cross the glia limitans they migrate and accumulate around Aβ plaques, possibly interacting with APCs (i.e. microglia, or peripheral monocytes or dendritic cells recruited towards CCL2) that present Aβ T-cell epitopes. Cytokines such as IFN-γ are secreted by the T cells and facilitate Aβ clearance either by brain endogenous microglia or by infiltrating microglia-like cells. (5) T cells secreting IFN-γ and/or neurotrophic factors stimulate neural precursor cell proliferation and differentiation.
