Figure 1.
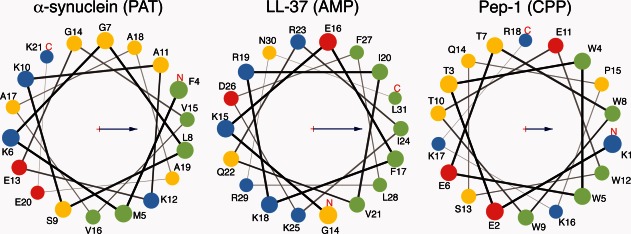
Helical wheel diagrams for three representative peptides discussed in this review. These are α-synuclein,8 which forms a PAT associated with disease progression in Parkinson's disease, LL-37,10 a human AMP, and Pep-1,11 a designed CPP. Members of these three classes of peptide generally possess an amphipathic structure, as seen here, with nonpolar and polar/charged residues segregated on opposite faces of an α-helix. This allows peptides to partition onto membranes, with the nonpolar regions residing in the acyl core and the polar regions contacting the lipid headgroups and solvent. Green circles represent nonpolar residues, yellow polar uncharged residues, red acidic residues, and blue basic residues. Arrows represent the hydrophobic moment of the shown helix. Helical wheel diagrams produced using MPEx.12
