Figure 6.
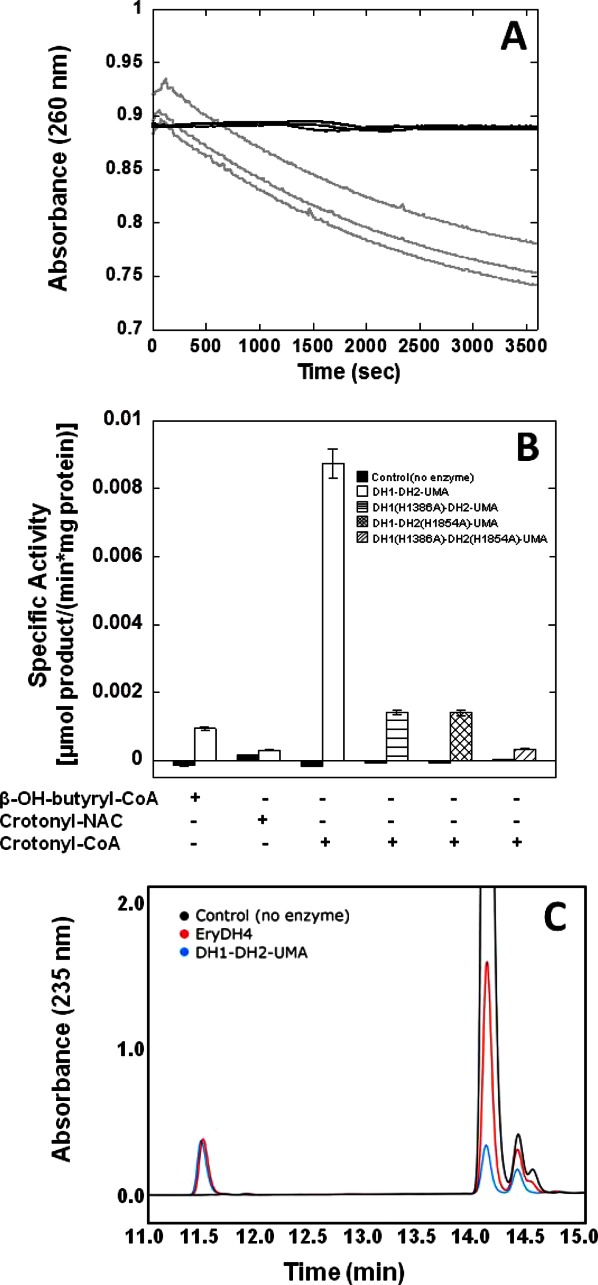
Enzymatic activity of DH1-DH2-UMA. (A) The decrease in absorbance at 260 nm was monitored as the substrate, crotonyl-CoA, was hydrated to β-hydroxybutyryl-CoA. The light gray line denotes the disappearance of the substrate in the presence of DH1-DH2-UMA and the dark gray lines denote the control with no enzyme. (B) The rates of the enzyme reaction were measured toward the substrates β-hydroxybutyryl-CoA, crotonyl-NAC, and crotonyl-CoA and converted to units of specific activity. Additionally, the activity of active site mutants DH1(H1386A)-DH2-UMA and DH1-DH2(H1854A)-UMA, as well as the activity of the double mutant was measured toward crotonyl-CoA. (C) The formation of hydrated product of crotonyl-pantetheine was confirmed by reverse phase HPLC. The black trace corresponds to the substrate alone with a major peak eluting at 14 min. In the presence of DH1-DH2-UMA (blue trace), the formation of a peak which elutes at 11.5 min can be appreciated. This peak is also observed in the presence of DH4 from the erythromycin gene cluster whose activity is well established (red trace).20
