Abstract
The ethanol Hedera helix plant extract was tested for its antiinflammatory properties. Intraperitoneal injections of 7.5 ml/kg wt ethanol extract showed antiinflammatory activity with 88.89% inhibition as compared to reference drug diclofenac, which showed 94.44% inhibition in formalin-induced paw oedema. As formalin-induced paw oedema closely resembles human arthritis, the antiarthritic property of ethanol extract of Hedera helix was also investigated. The visible reduction in arthritic symptoms by extract of Hedera helix suggests the potential of the plant extract against inflammation and arthritis.
Keywords: Arthritis, diclofenac, Hedera helix, inflammatory, intraperitoneal injections
Hedera helix (Linn) is common ivy of family Araliaceae found growing in the Darjeeling hills. It is an evergreen woody climber scaling the walls and covering the walls with a canopy of leaves. It is also grown as an ornamental plant. In folklore medicine, it is used for the cure of benign warts. Inflammation is a fundamental protective response. However, inflammation can be harmful in conditions such as life-threatening hypersensitive reactions to insect bites, drugs, toxins and in chronic diseases such as rheumatic arthritis, atherosclerosis, lung fibrosis and cancer.[1] Inflammation has been seen to accelerate cancer and chronic inflammation is regarded as an essential factor for the progression of neoplastic processes.[2] Hedera helix extract has been reported to have antioxidant properties,[3–6] antispasmodic properties,[7] antiallergic effects.[8] The effect of dry extracts on respiratory functions of children with chronic bronchial asthma[9–10] and its antitumour activities has been reported.[11–16] In the quest for seeking new herbal sources for the treatment of inflammation and cancer, in the present study, Hedera helix was tested for its antiinflammatory properties using 2% formalin for induction of inflammation. Antiinflammatory experiments were carried out using diclofenac as the reference drug. The antiarthritic property of Hedera helix extract has also been investigated.
Swiss Albino mice (6-8 week old) of both sexes were used in the study. Animals of approximately equal age and weight were used for experimental and control groups. All animal experiments were carried out according to the guidelines of the Animal Ethics Committee (Reg no. 840/ac/04/CPCSEA).
For the preparation of Hedera extract, leaves of the plant were collected and was identified at the Botany Department of St. Joseph’s College, Darjeeling. The leaves were washed with water. After soaking away excess water, 10 g of the leaves were taken and crushed to a paste in a mortar and pestle. Fifteen millilitres of absolute alcohol were added and kept in a refrigerator at 4° for 12 h. The extract was then filtered through Whatman filter paper no. 1; then the filtrate was filtered through Millipore filter and the final solution obtained was stored at 4° for further use.[17]
Animals were divided into five groups of eight mice each (Group A, B, C, D and E). Twenty minutes before induction of inflammation, Group A mice were used as control and each animal of Group A received phosphate buffered saline (PBS) intraperitoneally (i.p.) only (3 ml/kg). Group B, C and D test mice received ethanol Hedera extract 2.5-7.5 ml/kg wt (25 μl, 50 μl and 75 μl per animal) i.p., respectively. Group E animals received diclofenac as reference drug 100 mg/kg. Diclofenac was used because it is a nonsteriodal antiinflammatory drug. Diclofenac has been reported to suppress inflammation induced by various phlogistic agents in experimental animal models. Diclofenac is commonly employed in the treatment or management of rheumatoid arthritis, osteoarthritis and ankylosing spondylitis and for its antiinflammatory and analgesic effects. Diclofenac reduces inflammation, swelling and arthritic pain by inhibiting prostaglandin synthesis and/or production. The drug also affects polymorphonuclear leukocyte functions in vitro, thereby reducing chemotaxis, superoxide toxic radical formation, oxygen-derived free radical generation and neutral protease production.[18]
Freshly prepared 2% formalin was used as the oedematogenic agent. Twenty minutes after administration of PBS in control animals and after injection of various doses of plant extract and reference drug in experimental animals, each animal was injected with 20 μl, of formalin directly on subplantar region of left hind paw by exmire microsyringe (ITO Corporation, Tokyo, Japan).[19] Formalin-induced paw oedema is one of the most suitable test procedures to screen chronic antiinflammatory agents, as it closely resembles human arthritis.[20] The nociceptive effect of formalin is also biphasic as it acts as an early neurogenic component followed by a later tissue-mediated response.[21] The antiinflammatory response of Hedera extract on formalin-induced paw oedema suggests the usefulness of Hedera extract in the treatment of inflammation-associated diseases like arthritis.
Percent inhibition were calculated as, increase in paw thickness in control and experimental animals is Pc=Pt−Po and PT=Pt−Po, respectively and % inhibition=([Pc−PT]/Pc)×100, where Pt is paw thickness at time t, Po is initial paw thickness, Pc is increase in paw thickness of control animals, PT is the increase in paw thickness of the treatment animals.[19]
Pedal inflammation was evident 5-8 min after formalin injection. The paw thickness was measured using Vernier callipers before and after formalin treatment. The thickness of paw was recorded at 30, 60, 90, 120, 150 and 180 min after formalin injection. The data were statistically analysed using Student’s t test and P values less than 0.001 were considered significant. All data were represented as mean±SD.[22,23]
After induction, paw thickness increased upto 90 min of induction and then decreased steadily in treatment animals as compared to control animals where the paw thickness increased steadily. Within 180 min, the paw thickness came back to nearly normal size in diclofenac and 75 μl Hedera extract treated animal (Table 1).
Table 1.
EFFECT OF ETHANOL EXTRACT OF HEDERA HELIX AND DICLOFENAC ON LEFT PAW OEDEMA INDUCED BY FORMALIN
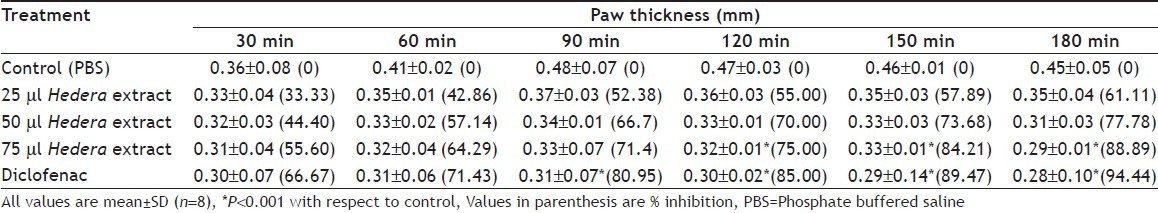
Percent inhibition was highest with 88.89% inhibition with 75 μl Hedera extract at 180 min after induction of inflammation as compared to reference drug diclofenac, which was 94.44% inhibition at 180 min after induction. Lower doses of 25 μl and 50 μl Hedera extract also showed 61.11% and 77.7% inhibition, respectively. The antiinflammatory effect of 75 μl alcoholic Hedera extract nearing the effect of diclofenac suggests its strong antiinflammatory property. However, the mice could not tolerate peritoneal injections of more than 75 μl ethanol Hedera extract. Further works using concentrated strengths of Hedera extract in ethanol tolerable by the animals needs to be carried out in future.
Control animals with oedema induced with formalin were observed for 7 days. Arthritic properties with swelling of joints of left leg and tail were observed. These animals with swollen joints were taken for further arthritic treatment with Hedera extract. Erythrocyte sedimentation rate (ESR) of blood was carried out in these animals with swollen joints. Blood was collected by tail vein puncture of the animals with a 1 ml syringe. The blood was anticoagulated with 12% ethylenediaminetetraacetic acid and diluted 1:1 with sodium citrate. ESR was measured using Westergren’s method with pipette calibrated in mm from 0 to 200. ESR of normal animals was regarded as control. ESR acts as a guide to the progress of a disease. It is useful as an aid in differential diagnosis.
For the treatment of arthritic symptoms, 75 μl Hedera extract was injected at paw once daily for next 7 days. After 7 days treatment with Hedera extract, blood was again collected by tail vein puncture and whole trunk collection and ESR carried out.
Injection of 75 μl Hedera extract to the paws with swollen joints once daily for 7 days showed marked improvement on the swelling suggesting that Hedera helix is a potent herb for the treatment of arthritis in animals. ESR studies show that ESR values before Hedera extract treatment is 3.4 mm/h as compared to normal and after Hedera treatment (2.5 mm/h) (fig. 1)indicating progression towards recovery and decrease in inflammation in the body.
Fig. 1.
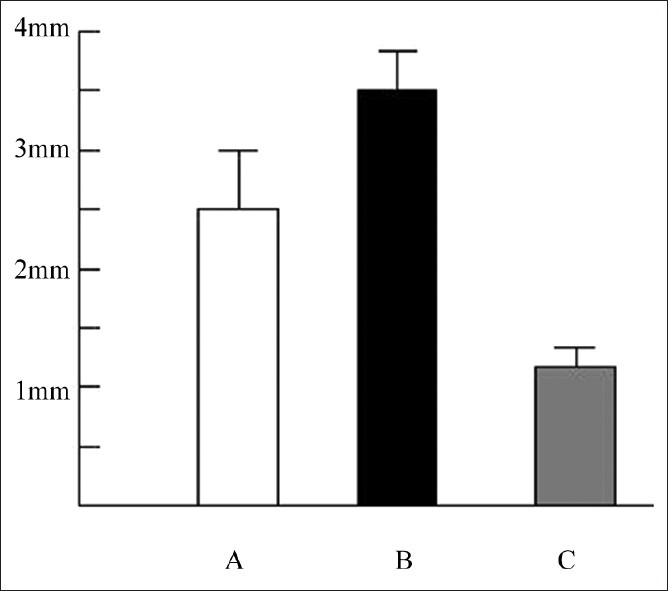
Erythrocyte sedimentation rate values of blood after 2 h.
A-After treatment;B-Before treatment; C- Control/normal
Though, some work in plant extraction and identification and synthesis of the various components of the plant is being carried out[24,25] especially, for its antitumour activity, the exact component that brings about the antiinflammatory, analgesic and antiarthritic property needs to be isolated and tested. A new horizon for herbal treatment has opened up and Hedera helix would be a cost-effective and a potent herbal medicine for the treatment of inflammation and arthritis.
ACKNOWLEDGEMENTS
The author would like to thank Prof. Ashim K. Chakravarty, Emeritus Professor, Centre for Life Sciences, NBU and Mr. Tamal Mazumdar for extending their help in the course of the study. The author would also like to thank the UGC for granting the Minor Project and financial help under which this work was carried out.
Footnotes
Rai: The Antiinflammatory and Antiarthritic Properties of Hedera helix
REFERENCES
- 1.Collins T. Acute and chronic inflammation. In: Cotran RS, Kumar V, Collins T, editors. Textbook of Robbins Pathologic Basis of Diseases. Philadelphia: W. B. Sounders Company; 1999. p. 50. [Google Scholar]
- 2.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Süleyman H, Mshvildadze V, Gepdiremen A, Elias R. Acute and chronic antiinflammatory profile of the ivy plant, Hedera helix, in rats. Phytomedicine. 2003;10:370–4. doi: 10.1078/0944-7113-00260. [DOI] [PubMed] [Google Scholar]
- 4.Gepdiremen A, Mshvildadze V, Süleyman H, Elias R. Acute antiinflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine. 2005;12:440–4. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Gepdiremen A, Mshvildadze V, Süleyman H, Elias R. Acute and chronic antiinflammatory effects of Hedera colchica in rats. J Ethnopharmacol. 2004;94:191–5. doi: 10.1016/j.jep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Gülçin I, Mshvildadze V, Gepdiremen A, Elias R. Antioxidant activity of saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med. 2004;70:561–3. doi: 10.1055/s-2004-827158. [DOI] [PubMed] [Google Scholar]
- 7.Trute A, Gross J, Mutschler E, Nahrstedt A. In vitro antispasmodic compounds of the dry extract obtained from Hedera helix. Planta Med. 1997;63:125–9. doi: 10.1055/s-2006-957627. [DOI] [PubMed] [Google Scholar]
- 8.Jones JM, White IR, White JM, McFadden JP. Allergic contact dermatitis to English ivy (Hedera helix): A case series. Contact Dermatitis. 2009;60:179–80. doi: 10.1111/j.1600-0536.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 9.Bolbot Y, Prokhorov E, Mokia S, Yurtseva A. Comparing the efficacy and safety of high concentrate (5-7.5:1) ivy leaves extract and acetylcysteine for treatment of children with acute bronchitis. Drugs Ukraine. 2004;11:1–4. [Google Scholar]
- 10.Hofmann D, Hecker M, Völp A. Efficacy of dry extract of ivy leaves in children with bronchial asthma: A review of randomized controlled trials. Phytomedicine. 2003;10:213–20. doi: 10.1078/094471103321659979. [DOI] [PubMed] [Google Scholar]
- 11.Villani P, Orsiere T, Sari-Minodier I, Bouvenot G, Botta A. In vitro study of antimutagenic activity of alpha hederin. Ann Biol Clin (Paris) 2001;59:285–9. [PubMed] [Google Scholar]
- 12.Elias R, De Méo M, Vidal-Ollivier E, Laget M, Balansard G, Dumenil G. Antimutagenic activity of some saponins isolated from Calendula officinalis L., C. arvensis L. and Hedera helix L. Mutagenesis. 1990;5:327–31. doi: 10.1093/mutage/5.4.327. [DOI] [PubMed] [Google Scholar]
- 13.Arcuceanu RV, Istudor V. Pharmacologically active natural compounds for lung cancer. Altern Med Rev. 2004;9:402–19. [PubMed] [Google Scholar]
- 14.El-Merzabani MM, El-Aaser AA, Attia MA, El-Duweini AK, Ghazal AM. Screening system for Egyptian plants with potential antitumour activity. Planta Med. 1979;36:150–5. doi: 10.1055/s-0028-1097255. [DOI] [PubMed] [Google Scholar]
- 15.Bun SS, Elias R, Baghdikian B, Ciccolini J, Ollivier E, Balansard G. Alpha-hederin potentiates 5-FU antitumor activity in human colon adenocarcinoma cells. Phytother Res. 2008;22:1299–302. doi: 10.1002/ptr.2483. [DOI] [PubMed] [Google Scholar]
- 16.Barthomeuf C, Debiton E, Mshvildadze V, Kemertelidze E, Balansard G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002;68:672–5. doi: 10.1055/s-2002-33807. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty AK, Yasmin H, Das SK. Two-way efficacy of alcoholic turmeric extract: Stimulatory for murine lymphocytes and inhibitory for fibrosarcoma cells. Pharm Biol. 2004;42:217–24. [Google Scholar]
- 18.Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zinziber officinale (roscoe) rhizomes (zingiberaceae) in mice and rats. Phytother Res. 2006;20:764–72. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 19.Nitha B, Meera CR, Janardhanan KK. Antiinflammatory and antitumour activities of cultured mycelium of morel mushroom, Morchella esculenta. Curr Sci. 2007;92:235–9. [Google Scholar]
- 20.Greenwald RA. Animal model for evaluation of arthritic drugs. Methods Find Exp Clin Pharmacol. 1991;13:75–83. [PubMed] [Google Scholar]
- 21.Wheeler-Aceto H, Cowan A. Neurogenic and tissue-mediated components of formalin-induced edema: Evidence for supraspinal regulation. Agents Actions. 1991;34:264–9. doi: 10.1007/BF01993299. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RA, Yates F. Statistical Tables for Biological Agricultural and Medical Research. London: Longman; 1974. pp. 1–146. [Google Scholar]
- 23.Ghose KC, Manna B. Practical Zoology. Kolkata: New Central Book Agency; 1996. pp. 411–8. [Google Scholar]
- 24.Chopra RN, Chopra IC, Varma BS. Supplement to Glossary of Indian Medicinal Plant. New Delhi: Publication and Information Directorate CCSIR; 1969. p. 35. [Google Scholar]
- 25.Daniel M. Chemistry and Properties. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.; 2006. Medicinal plants; p. 36. [Google Scholar]


