Abstract
Puccinia psidii, the cause of a disease today commonly referred to as Myrtle rust, is considered a high priority quarantine threat globally. It has a wide host range in the Myrtaceae and it is feared that it may result in significant damage to native ecosystems where these plants occur. The fungus is also of considerable concern to plantation forestry industries that propagate Australian Eucalyptus species. In May 2013, symptoms of a rust disease resembling those of P. psidii were observed on an ornamental Myrtaceous shrub in a garden in South Africa. The fungus was identified based on DNA sequence data of the ITS and 5.8S nrRNA gene regions and here we report, for the first time, the presence of P. psidii in Africa.
Keywords: Guava rust, Eucalyptus rust, Myrtus communis, Pucciniaceae, Uredo rangellii
INTRODUCTION
Puccinia psidii (Uredinales, Pucciniaceae) has been considered as an important quarantine threat to many countries (Glen et al. 2007). It was first described from native guava (Psidium guajava) in Brazil in 1884 and gained notoriety when it was found to infect various other members of the Myrtaceae, an unusual feature for most rust fungi (Coutinho et al. 1998, Glen et al. 2007, Carnegie et al. 2010a, Morin et al. 2012). Puccinia psidii became particularly prominent in the literature when it was found causing disease on non-native Eucalyptus species in Brazil (Jollify 1944) and it was rapidly considered as a significant threat to the commercial production of Eucalyptus species globally (Coutinho et al. 1998). It was also feared to threaten the survival of native Myrtales in countries such as Australia where this order represents a mega-diverse group of plants (Glen et al. 2007, Morin et al. 2012).
Since the first report of P. psidii in Brazil, the rust has been recorded in several countries of South and Central America, including the Caribbean (Coutinho et al. 1998, Glen et al. 2007, Graça et al. 2011, Morin et al. 2012). It has moved, increasingly rapidly, to new environments including California, Florida and Hawaii in the USA (Marlatt & Kimbrough 1979, Rayachhertry et al. 1997, Uchida et al. 2006), Japan (Kawanishi et al. 2009), Australia (Carnegie et al. 2010), and China (Zhuang & Wei 2011). Rusts in the guava/eucalyptus rust complex are native to South and Central America (Alfenas et al. 2005) and are feared because of their wide host range in Myrtaceae, including over 125 species (Morin et al. 2012). Thus, the recent appearance of P. psidii in Australia has resulted in many studies to consider its likely long-term impact (Carnegie & Cooper 2011, Morin et al. 2012, Kriticos et al. 2013).
Several common names have been used for the disease caused by P. psidii. That it was first found on guava led to it being known as Guava rust, but its appearance on more commercially important Eucalyptus species led to it commonly being referred to as Eucalyptus rust. When it first appeared in Australia, there was debate regarding its taxonomy and whether the fungus infecting a wide range of trees in Myrtaceae might not be the rust that had been described as Uredo rangelii (Carnegie et al. 2010, Carnegie & Cooper 2011). While there are clearly taxonomic issues relating to this fungus that remain to be resolved, such as that it is not phylogenetically related to other members of the genus Puccinia (M. Wingfield & W. Maier, unpubl. data), the disease caused by the fungus currently treated as P. psidii is best referred to as Myrtle rust. This captures the occurrence of the pathogen on a very wide host range including, numerous genera and species of Myrtales.
In May 2013, an ornamental Myrtus communis plant growing in a residential garden in the KwaZulu-Natal province of South Africa was discovered showing typical symptoms of infection by P. psidii. The aim of this study was to identify the fungus using DNA sequence data and to determine whether one of the globally most important invasive alien plant pathogens might have entered South Africa, which would represent the first confirmed record of this pathogen on the African continent.
MATERIALS AND METHODS
Leaves and shoots were taken from the infected Myrtus communis plant, collected in brown paper bags, and transported to the laboratory for study. The plant showed typical symptoms of infection by Puccinia psidii. These included leaf spots and cankers on young shoots/petioles (Fig. 1). Infected tissues were covered with yellow urediniospores. Spores were collected directly from infected material and used for morphological and DNA sequence studies (Table 1).
Fig. 1.
Puccinia psidii on Myrtus communis in South Africa. A. Leaf spot on Myrtus communis. B. Yellow masses of urediniospores covering a dying M. communis shoot tip. C–D. Urediniospores with echinilations and smooth pathes (tonsures).
Table 1. List of Puccinia isolates used in DNA sequence analyses.
| Species | Origin | GenBank Accession nr. | Host |
|---|---|---|---|
| Puccinia psidii | Australia | HM448900 | Agonis flexuosa |
| Brazil | AJ536601 | Psidium guajava | |
| Brazil | AJ421801 | Eugenia uniflora | |
| Brazil | AJ421802 | Melaleuca quinquenervia | |
| Colombia | EU711423 | Syzygium jambos | |
| Hawaii | EF599768 | Metrosideros polymorpha | |
| Hawaii | EU071045 | Melaleuca quinquenervia | |
| Florida | AJ535659 | Pimenta dioca | |
| Japan | AB470483 | Metrosideros polymorpha | |
| South Africa | KF220289 | Myrtus communis | |
| South Africa | KF220290 | M. communis | |
| South Africa | KF220291 | M. communis | |
| South Africa | KF220292 | M. communis | |
| South Africa | KF220293 | M. communis | |
| Uruguay | EU348742 | Eucalyptus grandis | |
| Uruguay | EU348743 | E. globulus | |
| P. cygnorum | EF490601 | Kunzea ericifolia | |
| P. hordei | AF511086 | n/a | |
| P. recondita | AF511082 | Triticum turgidum |
For DNA sequence studies, urediniospores were scraped from the surface of infected material into 18 μL sterile SABAX water. The SABAX water containing urediniospores were incubated at 94 °C for 10 minutes and used as template in subsequent reactions for amplification of the ITS1, 5.8S and ITS2 gene regions of the Internally Transcribed Spacer regions of the nuclear rDNA. Amplification reactions were performed in a final reaction volume of 25 μL containing: 5 μL 5× MyTaq™ Reaction Buffer (Bioline, London), 0.2 mM of each of the universal primers ITS 1-F (Gardes & Bruns 1993) and ITS 4 (White et al. 1990) and 1 U of MyTaq™ DNA polymerase. The PCR conditions were as follows: Initial denaturation at 94 °C for 3 min followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 53 °C for 1 min, and elongation at 72 °C for 1 min. A final elongation step at 72 °C for 10 min followed. Products were separated using gel electrophoresis and visualised using GelRed™ (Biotium, CA).
PCR Amplification products were purified using the Zymo research DNA Clean & Concentration™ - 5 kit (CA). Fragments were sequenced, using forward and reverse primers as described above, using the ABI Prism® Big DyeTM Terminator 3.0 Ready Reaction Cycle sequencing Kit (Applied Biosystems, Foster City, CA). Sequences were determined with an ABI PRISM™ 3100 Genetic Analyzer (Applied Biosystems). DNA sequences of opposite strands were edited and consensus sequences obtained using CLC Main workbench v. 6.1 (CLC Bio, www.clcbio.com) and MEGA v. 5 (Tamura et al. 2011). Sequences obtained were submitted to NCBI’s GenBank (http://www.ncbi.nlm.nih.gov/Genbank/index.html) with accession numbers KF220289 – KF220293.
Sequences obtained for the rust fungus from South Africa were subjected to a Blastn search on the NCBI database (http://www.ncbi.nlm.nih.gov) and thereafter incorporated into a dataset of closely related sequences for phylogenetic analyses. After online alignment using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/), the programme MEGA v. 5.1 (Tamura et al. 2011) was used to check the alignments and conduct a Maximum Likelihood analysis of the data set. Puccinia cygnorum (EF490601), P. hordei (AF511086), and P. recondita (AF511082) were used as outgroup species in the analyses.
Measurements of 20 urediniospores were made using a Zeiss Axioscop compound microscope and photographic images were captured using a Zeiss Axiocam MRc digital camera and the AxioVision v. 4.8 (Carl Zeiss) software. Scanning electron micrographs (SEM) were obtained directly from urediniospores scraped from infected material using a JSM-840 SEM (JEOL, Tokyo) at 5 kV and images captured with Orion v. 6.60.4 (E.L.I. s.p.r.l., Brussels, Belgium).
RESULTS
The infected Myrtus communis plant showed symptoms of leaf spot, with red margins, and the presence of abundant yellow spore masses on young shoots and leaves (Fig. 1). Infection resulted in the death of shoots and leaves. Only urediniospores were observed on the plant. These ranged in size from 15–20 (av = 19) × 12–16 (av = 14) μm. SEM of the urediniospores revealed the presence of tonsures (smooth patches) on the surfaces of some spores (Fig. 2).
Fig. 2.
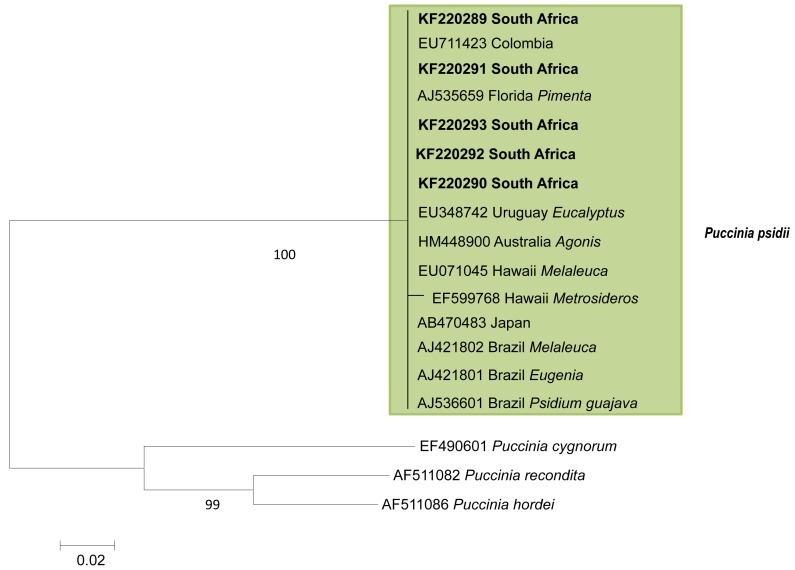
Phylogenetic tree of ITS sequence data showing identity of South Africa Puccinia psidii isolates. Maximum likelihood tree based on 626 bp from 17 taxa. Numbers below branches indicate bootstrap support values.
Amplification reactions of the ITS and 5.8S gene regions resulted in fragments of ~700 bp in length. The Blast search on the NCBI database showed that the rust fungus from South Africa (KF220289 – KF220293) was most closely related to Puccinia psidii. Subsequent comparisons using ML in MEGA, of a dataset comprising 17 sequences (Table 1) of 626 bp in length, showed that there were no differences in the ITS sequences between the South African collection and those from other parts of the world. All P. psidii isolates grouped together in a single clade, separately from the other Puccinia species included in the analyses.
DISCUSSION
This is the first confirmed report of the Myrtle rust pathogen, Puccinia psidii, from South Africa. There have been two previous reports of a rust fungus on Eucalyptus in South Africa (Knipscheer & Crous 1990, Maier et al. 2010), but both were morphologically different to P. psidii. The present study provides robust evidence that P. psidii is now present in South Africa. This is an important discovery and it is one that is of considerable concern, especially as the species is unrecorded elsewhere in Africa.
The discovery of P. psidii in South Africa is significant to both the commercial plantation forestry industry, as well as the conservation of native plants and associated ecosystems. The impact of P. psidii on plantation grown Eucalyptus species has been shown in several previous studies (Tommerup et al. 2003, Graça et al. 2011, Silva et al. 2013). More recently, a number of studies have shown the broad host range of the pathogen on other Myrtaceae and the fungus has been described as a significant threat to native ecosystems in Australia (Morin et al. 2012). Importantly, a study has also shown that native South African Heteropyxis natalensis (Myrtales, Heteropyxidaceae) is highly susceptible to infection by P. psidii (Alfenas et al. 2005).
The majority of P. psidii hosts reside in the subfamily Myrtoideae of the Myrtaceae (Morin et al. 2012). The susceptibility, in greenhouse studies, of South African H. natalensis in Heteropyxidaceae, clearly shows the potential damage that the pathogen might pose to other families in Myrtales. South Africa is home to three families in Myrtales (Palgraves & Palgraves 2002), including species that are endemic and thus of significant conservation importance. Of these, five genera (Eugenia, Heteropyxis, Memecylon, Syzygium, and Warneckeae) occur in the KwaZulu-Natal Province where P. psidii has now been detected. Clearly, much work has yet to be done to consider the potential impact that P. psidii might have in South Africa. It is known that P. psidii has considerable race specialisation (Coelho et al. 2001, Aparecido et al. 2003) and it will be necessary to determine the host range of the fungus now present, but known only on a single host species from a single locality in South Africa.
The fungus on Myrtus communis in South Africa is morphologically similar to P. psidii from elsewhere in the world. Urediniospores of the South African collection were, however, smaller than those reported for P. psidii from Uruguay (Perez et al. 2011) and for Uredo rangelii (Simpson et al. 2006). SEM showed the presence of typical spines as well as tonsures on the urediniospores. There have been previous arguments that these tonsures are characteristic of U. rangelii, and not P. psidii (Simpson et al. 2006, Carnegie et al. 2010a). However, they are common on the rust now considered to be P. psidii in Australia (Carnegie et al. 2011) and this is also true for collections that infect Eucalyptus and native Myrtaceae in Uruguay (Carnegie et al. 2010, Perez et al. 2011). Although P. psidii might represent a suite of related cryptic species (Wingfield, personal observation), in-depth studies are needed to elucidate this question.
There are relatively limited options to manage the P. psidii infection. Eradication of this new invasion is unlikely to be effective because rust fungi produce abundant air-borne spores, and will be highly dependent on rapid action. Selection of resistant plant material will be the most durable approach. For example, considerable variation has been identified in susceptibility of Eucalyptus genotypes to infection by P. psidii (Carvalho et al. 1998, Junghans et al. 2003, Silva et al. 2012), which implies that it will be possible to restrict the damage that might occur in commercial plantation situations. The deployment of resistant native Myrtaceae, if present, will, however, be more complicated than that of a commercial crop such as Eucalyptus in South Africa. Dealing with the disease in native ecosystems will be much more complex. Therefore, great effort should be made to slow the movement of the pathogen into native ecosystems, particularly in areas such as the Western Cape, where only a single, endemic species of Myrtaceae (Metrosideros angustifolia) occurs. In Australia, Puccinia psidii was described as “the pinnacle of pathogens we wanted to keep out of Australia” (Dayton & Higgins 2011). Its appearance in South Africa is likely to have substantial negative long-term consequences for both forestry and plant conservation. South Africa is also likely to now provide the bridge for P. psidii to move northwards in Africa.
Acknowledgments
We thank members of the Tree Protection Co-operative Programme (TPCP), DST/NRF Centre of Excellence in Tree Health Biotechnology (CTHB) and the University of Pretoria for funding and facilities to undertake this work. We also thank Mr. Alan Hall of the microscopy unit of the University of Pretoria for the SEM photos of the urediniospores.
REFERENCES
- Alfenas AC, Zauza EAV, Wingfield MJ, Roux J, Glen M. (2005) Heteropyxis natalensis, a new host of Puccinia psidii rust. Australasian Plant Pathology 34: 285–286 [Google Scholar]
- Aparecido CC, Figueiredo MB, Furtado EL. (2003) Groups of physiological variability in Puccinia psidii populations. Summa Phytopathologica 29: 234–238 [Google Scholar]
- Booth TH, Old KM, Jovanovic T. (2000) A preliminary assessment of high risk areas for Puccinia psidii (Eucalyptus rust) in the Neotropics and Australia. Agriculture, Ecosystems and Environment 82: 295–301 [Google Scholar]
- Carnegie AJ, Lidbetter JR, Walker J, Horwood MA, Tesoriero L, Glen M, Priest M. (2010) Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australasian Plant Pathology 39: 463–466 [Google Scholar]
- Carnegie AJ, Cooper K. (2011) Emergency response to the incursion of an exotic myrtaceous rust in Australia. Australasian Plant Pathology 40: 346–359 [Google Scholar]
- Carvalho ADO, Alfenas AC, Maffia LA, Carmo MGF. (1998) Resistance of Eucalyptus species, progenies and provenances to Puccinia psidii. Pesquisa Agropecuária Brasileira 33: 139–147 [Google Scholar]
- Coelho L, Alfenas AC, Ferreira FA. (2001) Physiologic variability of Puccinia psidii – the rust of Eucalyptus. Summa Phytopathologica 27: 295–300 [Google Scholar]
- Coutinho TA, Wingfield MJ, Alfenas AC, Crous PW. (1998) Eucalyptus rust – a disease with the potential for serious international implications. Plant Disease 82: 819–825 [DOI] [PubMed] [Google Scholar]
- Ferreira FA. (1981) Ferrugem do eucalipto ocorrencia, temperatura para geminatio de uredosporos produlfao de teliosporos, hospedeiro alternativo e resistencia. Fitopatologia Brassileira 6: 603–604 [Google Scholar]
- Dayton L, Higgins E. (2011) Myrtle rust “biggest threat to ecosystem”. The Australian, 9 April 2011, http://www.theaustralian.com.au/news/health-science/myrtle-rust-biggest-threat-to-ecosystem/story-e6frg8y6-1226036247221 [Google Scholar]
- Glen M, Alfenas AC, Zauza EAV, Wingfield MJ, Mohammed C. (2007) Puccinia psidii, a threat to the Aus‐tralian environment and economy – a review. Australasian Plant Pathology 36: 1–16 [Google Scholar]
- Graça RN, Aun CP, Guimarães LMS, Rodrigues BVA, Zauza EAV, Alfenas AC. (2011) A new race of Puccinia psidii defeats the Ppr-1 resistance gene in Eucalyptus grandis. Australasian Plant Pathology 40: 442–447 [Google Scholar]
- Graça RN, Alfenas AC, Ross-Davis AL, Klopfenstein NB, Kim M-S, Peever TL, Cannon PG, Uchida JY, Kadooka CY, Hauff RD. (2011) Multilocus genotypes indicate differentiation among Puccinia psidii populations from South America and Hawaii. In: Proceedings of the 58th Annual Western International Forest Disease Work Conference; 2010 October 4–8; Valemount, BC: 131–134 Flagstaff, AZ: US Forest Service, AZ Zone Forest Health [Google Scholar]
- Joffily J. (1944) Ferrugem do eucalipto. Bragantia 4: 475–487 [Google Scholar]
- Junghans DT, Alfenas AC, Brommonschenkel SH, Oda S, Mello EJ, Grattapaglia D. (2003) Resistance to rust (Puccinia psidii Winter) in Eucalyptus: mode of inheritance and mapping of a major effect locus with RAPD markers. Theoretical and Applied Genetics 108: 175–180 [DOI] [PubMed] [Google Scholar]
- Kawanishi T, Uemastu S, Kakishima M, Kagiwada S, Hamamoto H, Horie H, Namba S. (2009) First report of rust disease on ohia and the causal fungus in Japan. Journal of Genetic Plant Pathology 75: 428–431 [Google Scholar]
- Knipscheer NS, Crous PW. (1990) First record of a rust disease on Eucalyptus. Forestry News 90(2): 22–23 [Google Scholar]
- Kriticos DJ, Leriche A. (2008) The current and future potential distribution of guava rust, Puccinia psidii in New Zealand. [MAF Biosecurity new Zealand Technical Paper no.: 2009/28]. Wellington: Ministry of Agriculture and Forestry [Google Scholar]
- Kriticos DJ, Morin L, Leriche A, Anderson RC, Caley P. (2013) Combining a climatic niche model of an invasive fungus with its host species distributions to identify risks to natural assets: Puccinia psidii sensu lato in Australia. PLoS ONE 8: e64479 Doi:10.1371/journal.pone.0064479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Roux J, Wingfield BD, Coetzee MPA, Wingfield MJ. (2010) A new Eucalyptus rust from Mozambique and South Africa. Proceedings of the 4th International Rusts of Forest Trees Conference, Florence, Italy, 3–6 May 2010: 423 [Google Scholar]
- Marlatt RB, Kimbrough JW. (1979) Puccinia psidii on Pimenta dioica in south Florida. Plant Disease Reporter 63: 510–512 [Google Scholar]
- Morin L, Aveyard R, Lidbetter JR, Wilson PG. (2012) Investigating the host-range of the rust fungus Puccinia psidii sensu lato across tribes of the family Myrtaceae present in Australia. PLoS ONE 7: e35434, 10.1371/journal.pone.0035434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palgraves KC. [with Drummond RB, Moll EJ, eds] (2002) Palgrave’s Trees of Southern Africa. Cape Town: Struik Publishers [Google Scholar]
- Perez CA, Wingfield MJ, Altier NA, Simeto S, Blanchette RA. (2011) Puccinia psidii infecting cultivated Eucalyptus and native myrtaceae in Uruguay. Mycological Progress 10: 273–282 [Google Scholar]
- Silva PHM, Miranda AC, Moraes MLT, Furtado EL, Stape JL, Alvares CA, Sentelhas PC, Mori ES, Sebbenn AM. (2013) Selecting for rust (Puccinia psidii) resistance in Eucalyptus grandis in São Paulo State, Brazil. Forest Ecology and Management 303: 91–97 [Google Scholar]
- Simpson JA, Thomas K, Grgurinovic CA. (2006) Uredinales species pathogenic on species of Myrtaceae. Australasian Plant Pathology 35: 549–562 [Google Scholar]
- Tommerup IC, Alfenas AC, Old KM. (2003) Guava rust in Brazil – a threat the Eucalyptus and other Myrtaceae. New Zealand Journal of Forestry Science 33: 420–428 [Google Scholar]
- Zhuang J-Y, Wei S-X. (2011) Additional materials for the rust flora of Hainan Province, China. Mycosystema 30: 853–860 [Google Scholar]