Abstract
The genus Durotheca is introduced with D. depressa sp. nov., as type. Hypoxylon comedens is transferred to Durotheca, based on its morphology with further evidence from molecular phylogenetic studies; a combined β-tubulin and α-actin gene dataset. Theissenia cinerea is synonymized with D. comedens, and the type of Theissenia, T. pyrenocrata, is shown to occupy a basal, rather distant position in a monotypic clade in relation to sequenced taxa of Durotheca. This clade has an unresolved position in relation to the two informal subfamilies “Xylarioideae” and “Hypoxyloideae” within the Xylariaceae. New distributional data for D. comedens and T. pyrenocrata are presented, with the former found to be widespread in South-East Asia and the latter is reported as new from western Amazonia (Ecuador). One further species described in Theissenia, T. rogersii, is transferred to Durotheca, whilst T. eurima is accepted in Theissenia.
Keywords: α-actin, β-tubulin, Biodiversity, SEM, Thailand
INTRODUCTION
The genus Theissenia was introduced by Maublanc (1914) for Ustulina pyrenocrata. Læssøe (1994) accepted this genus within Xylariaceae, and Ju et al. (2003) recognized three species in their monographic treatment. Subsequently, another species was added and a phylogenetic analysis based on DNA sequences from two of the accepted taxa, but not the type species T. pyrenocrata, was provided (Ju et al. 2007). The inclusion in Xylariaceae was confirmed, and data were presented to show affinities within the subfamily “Hypoxyloideae”1, a position never previously proposed. Ju et al. (2003) had previously accepted, ad interim, a placement within the Xylariaceae based on a Nodulisporium-morph found in cultures of T. cinerea, but noted that the aleurisporous asexual morph found in T. eurima was not as expected for such a position. Furthermore, a similar asexual morph had been observed in T. pyrenocrata. Ju et al. (2003) also noted the extreme variability displayed among the four recognized species, such as the absence/presence of germ slits, surface ornamentation, and variability in ascospore wall thickness and asexual morphs. In our continuing studies on the biodiversity of Thai Xylariaceae, we have repeatedly encountered a fungus that we identified as Hypoxylon comedens based on a comparison with type material. Læssøe et al. (1989) excluded H. comedens from both Camillea and Hypoxylon, but were unable to suggest a revised placement. Also, the new material, including cultures, did not provide sufficient information to suggest a placement with confidence, not least due to our cultures failing to produce an asexual morph. Ju et al. (2003) shed some light on the situation, and we recognized that our fungus was a member of Theissenia in the sense of these authors.
Hypoxylon comedens was originally described from the Malaysian state Sarawak on Borneo (Cesati 1879), and has since been reported from China (Tai 1979, Zhuang 2001). Material determined as “Hypoxylon cf. comedens” was also reported from Mexico (San Martín González & Rogers 1993). Martin (1969) also published on a presumed H. comedens from Mexico, including data on the asexual state, but the material he used is evidently a species of Camillea. The cardinal features that excludes H. comedens from Hypoxylon, and its segregate genera, are the combination of the highly carbonized and large perithecia seated directly on the substrate, the clavate, deliquescing asci, and the peculiar spore shape and pale pigmentation. Furthermore, most collections yield no extractable pigments (with KOH) unlike most members of Hypoxylon.
Here we report on further morphological and molecular studies on material referable to H. comedens s.l. or Theissenia from Thailand and other parts of South-East Asia, and most importantly, on sequenced material of the type species of Theissenia from Ecuador.
MATERIAL AND METHODS
Sampling and culturing
Herbarium and genetic resource collection acronyms follow Thiers (2010). Field collected stromata of Hypoxlon comedens s.lat. and other xylariaceous species were taken to sites where isolation work could be carried out within a few hours. Within 2–3 d, ascospores germinated, and the resulting cultures were transferred to fresh plates (in Thailand) and later transferred to the collections at BCC. In Ecuador no culture work was carried out, and attempts to culture Theissenia pyrenocrata from dried material failed. All dried voucher collections are held at BBH, with cultures deposited in BCC (Thai material), or C and QCNE (Ecuadorian material).
Growth for DNA extraction
Cultures of Xylariaceae were grown on Potato Dextrose Agar (PDA) Petri plates. These plates were incubated at room temperature in darkness for 3–4 wk. A few small blocks of PDA with sterile or sporulating mycelium of each sample were taken from a plate and placed in 50 mL Sabouraud Dextrose Broth (Sigma; SDB) in 250 mL Erlenmeyer flasks, and incubated at 25 °C in darkness for 4 wk. The mycelial mass on SDB was then harvested over a sterile Whatman filter paper and washed with sterile, distilled water.
DNA extraction
Total DNA of each mycelial sample, or in the case of Theissenia pyrenocrata from perithecial contents, was extracted using Cetyltrimethyl-ammonium bromide (CTAB) following the procedure described in Mackill & Bonman (1995), with minor modifications (to adapt the procedure to the study of fungal material): Lyophilized mycelium (40–50 mg) was placed into a microcentrifuge tube and ground to powder. This mycelial powder was suspended in 700 μL of extraction buffer (NaCl 0.7 M; Tris-HCl 50 mM pH 8.0; EDTA 2 mM pH 8.0, 1 % CTAB) preheated to 65 °C. The suspension was thoroughly mixed and incubated for 1 h at 65 °C. After the suspension had cooled, 500 μL of chloroform/isoamyl alcohol (24:1 v/v) was added. The supernatant was gently mixed until an emulsion was obtained and centrifuged at 10 000 rpm for 20 min. The aqueous phase was transferred to a new sterile tube. A 10 % CTAB solution was added at one tenth of the volume of the aqueous phase and mixed. The supernatant was transferred to a new tube after a spin-down of 20 min. 700 μL of precipitation buffer (CTAB 1 %; Tris-HCl 50 mM pH 8.0; EDTA 10 mM pH 8.0) was then added to the supernatant, left at room temperature for 5–10 min and centrifuged. The aqueous phase was discarded and 300 μL of TEHS buffer (NaCl 1M; Tris-HCl 10 mM pH 8.0; EDTA 1 mM pH 8.0) was added to the pellet to remove the CTAB from the DNA. The pellet was treated with ribonuclease A, incubated at 37 °C for 30 min, followed by addition of 750 μL of cold absolute ethanol and centrifuged at 10 000 rpm for 20 min. The supernatant was discarded and the pellet was washed in 500 μL 70 % (v/v) ethanol and air-dried at room temperature. The DNA pellet was then dissolved in 50 μL TE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA pH 8.0).
PCR and sequencing
PCR amplification was done in a 50 μL volume consisting of 1× PCR buffer, 200 μM of each of the four dNTPs, 2.5 mM MgCl2, 1 U Taq DNA polymerase (Promega, Madison, Wisconsin) and 0.5 μM of each primer. Amplification of the partial β-tubulin gene and α-actin were done using the primer pairs T1/T22 (O’Donnell & Cigelnik 1997) and ACT-512F/ACT-783R (Carbone & Kohn 1999), respectively. Amplifications were performed using a MJ Research DNA Engine ALD1244 thermal cycler following the procedure described in Ju et al. (2007). PCR products were purified using a QIAquick PCR purification Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Purified PCR products were sent to Macrogen (Korea) for sequencing.
Sequence analysis
Each DNA sequence was checked for ambiguous bases, assembled using BioEdit v. 6.0.7 (Hall 2004), and submitted to GenBank (Table 1). Proofed sequences were then aligned using ClustalW (Larkin et al. 2007) incorporated in BioEdit v. 6.0.7 and alignments were refined by directed examination. Parsimony and Bayesian analyses were first carried out for each gene on individual datasets. Potential conflicts were assessed by comparing individual parsimony bootstrap trees. In case two different relationships for the same set of taxa were both supported by bootstrap values greater than 70 % from different genes, it was assumed that the incongruence was signicant (Wiens 1998). Parsimony (PAUP v. 4.0b10, Swofford 2002) and Bayesian (MrBayes v. 3.0, Huelsenbeck & Ronquist 2001) phylogenetic analyses were performed on the combined data set of the β-tubulin and α-actin genes. The maximum parsimony analysis was performed using the heuristic search, starting with trees obtained via 1000 random stepwise addition sequences, and tree-bisection-reconnection as the branch-swapping algorithm. All characters were given equal weights and gaps were treated as missing data. No topological constraints were enforced and the ‘Multrees’ option was in effect. Relative support for the branches was obtained from bootstrap proportions (BP) using 1000 heuristic searches using the aforementioned parsimony settings and 10 random sequence additions per bootstrap replicate. Prior to conducting the Bayesian analysis MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model. After the best nucleotide substitution model was determined for each gene partition and combined dataset, Bayesian analysis was conducted using MCMC using a GTR+I+G model. Four default chains were sampled every 100 generations and run for a total of 2 M generations. Bayesian posterior probabilities (PP) were calculated on the posterior distribution of trees excluding the initial set of burn-in trees.
Table 1.List of specimens used for the molecular phylogenetic study (Fig. 1).
| Taxon | Original code | Culture acc. no. | Origin | Locality/Collecting data (or origin in case of reference sequences retrieved from GenBank) |
GenBank Acc. no. |
|
|---|---|---|---|---|---|---|
| Alpha-Actin | Beta-Tubulin | |||||
| Ootheca comedens | XY00290 | BCC22770 | Thailand | Phu Hin Rong Kla National Park, Phitsanulok; BBH18116 | GQ160478 | GQ160486 |
| Ootheca comedens | XY00513 | BCC25014 | Thailand | Khao Nan National Park, Nakhon Si Thammarat; BBH25875 | GQ160479 | GQ160487 |
| Ootheca comedens | XY00531 | BCC25152 | Thailand | Khao Nan National Park, Nakhon Si Thammarat; BBH25876 | GQ160480 | GQ160488 |
| Ootheca comedens | XY00534 | BCC25155 | Thailand | Khao Ban That Wildlife Sanctuary Wildlife Sanctuary, Trang; BBH25877 | GQ160481 | GQ160489 |
| Ootheca comedens | XY00638 | BCC28080 | Thailand | Kaeng Krachan National Park, Phetchaburi; BBH19755 | GQ160482 | GQ160490 |
| Ootheca depressa | XY00402 | BCC23016 | Thailand | Doi Inthanon National Park, Chiang Mai; BBH18222 | GQ160483 | GQ160491 |
| Ootheca depressa | XY00619 | BCC28073 | Thailand | Doi Inthanon National Park, Chiang Mai; BBH19737 | GQ160484 | GQ160492 |
| Ootheca depressa | XY00636 | BCC28079 | Thailand | Doi Inthanon National Park, Chiang Mai (no specimen) | GQ160485 | GQ160493 |
| Theissenia pyrenocrata | TL-11480 | none | Ecuador | Orellana, TL-11480 (QCNE, C) | GQ247716 | GQ247717 |
| Amphilogia gyrosa | none | BCRC34145 | Taiwan | Ju & Hsieh 91123101 (HAST) (Ju et al. 2007) | EF025600 | EF025615 |
| Annulohypoxylon bovei var. microsporum | none | BCRC34012 | Taiwan | Ju & Hsieh 90081914 (HAST) (Hsieh et al. 2005) | AY951765 | AY951654 |
| Annulohypoxylon cohaerens | none | BCRC34013 | France | Fournier JF-03041 (Hsieh et al. 2005) | AY951766 | AY951655 |
| Annulohypoxylon moriforme var. microdiscus | none | BCRC34018 | Taiwan | Ju & Hsieh 90080807 (HAST) (Hsieh et al. 2005) | AY951769 | AY951660 |
| Annulohypoxylon nitens | none | BCRC34021 | Taiwan | Guu 91022108 (HAST) (Hsieh et al. 2005) | AY951772 | AY951663 |
| Annulohypoxylon squamulosum | none | BCRC34022 | Taiwan | Holotype (HAST), see Ju et al. (2004) as Hypoxylon squamulosum. and Hsieh et al. (2005) | AY951774 | AY951665 |
| Biscogniauxia anceps | none | BCRC34029 | France | Candoussau (Rogers et al. 1996, (Hsieh et al. 2005) | AY951783 | AY951671 |
| Biscogniauxia arima | none | BCRC34030 | Mexico | Isotype (Ju et al.. 1998, Hsieh et al. 2005) | AY951784 | AY951672 |
| Biscogniauxia capnodes | none | BCRC34032 | Taiwan | Ju 77031509 (Ju et al. 1998, (Hsieh et al. 2005) | AY951787 | AY951675 |
| Biscogniauxia cylindrispora | none | BCRC33717 | Taiwan | Holotype (Ju & Rogers 2001, (Hsieh et al. 2005) | AY951791 | AY951679 |
| Biscogniauxia latirima | none | BCRC34036 | Taiwan | Ju & Hsieh 90080703 (HAST) (Hsieh et al. 2005) | AY951795 | AY951683 |
| Biscogniauxia mediterranea | none | BCRC34037 | France | Candoussau 366 (Ju et al. 1998, Hsieh et al. 2005) | AY951796 | AY951684 |
| Biscogniauxia philippinensis var. microspora | none | BCRC33720 | Taiwan | Ju 89041101 (HAST) (Ju & Rogers 2001, Hsieh et al. 2005) | AY951797 | AY951685 |
| Biscogniauxia simplicior | none | BCRC34038 | France | Candoussau 5354A (Ju et al. 1998, Hsieh et al. 2005) | AY951798 | AY951686 |
| Cryphonectria macrospora | none | BCRC34146 | Taiwan | Ju & Hsieh 94031513 (HAST) (Ju et al. 2007) | EF025587 | EF025618 |
| Daldinia caldariorum | none | BCRC34042 | Taiwan | Chen 957 (HAST) (Hsieh et al. 2005) | AY951802 | AY951690 |
| Daldinia vernicosa | none | BCRC34048 | Germany | Wollweber 2899 (Ju et al. 1999 and Bitzer et al. 2008; as D. fissa); now deposited in KR 0026318 | AY951809 | AY951697 |
| Daldinia loculata | none | KC1525 (Kew) | UK | K[M] 24541 (Stadler et al. 2001, Hsieh et al. 2005) | AY951810 | AY951698 |
| Hypoxylon rubiginosum | none | BCRC34116 | UK | J.D. Rogers (Ju & Rogers 1996, (Hsieh et al. 2005)) | AY951862 | AY951751 |
| Hypoxylon shearii var. minor | none | BCRC34093 | Mexico | Isotype (WSP) (San Martin et al. 1999, (Hsieh et al. 2005)) | AY951864 | AY951753 |
| Kretzschmaria clavus | none | BCRC34147 | French Guiana | Huhndorf 803 (WSP) (Rogers & Ju 1998, Hsieh et al. 2009) | EF025596 | EF025611 |
| Kretzschmaria lucidula | none | BCRC34148 | French Guiana | Huhndorf 677 (Rogers & Ju 1998, Hsieh et al. 2009) | EF025595 | EF025610 |
| Kretzschmaria megalospora | none | N / A | Malaysia | M. Whalley FH 64-97 (JDR) (Hsieh et al. 2009) | EF025594 | EF025609 |
| Nemania illita | none | BCRC34150 | USA | Missouri, Columbus, S.J. Tsai (JDR) (Hsieh et al. 2009) | EF025593 | EF025608 |
| Nemania primolutea | none | BCRC34151 | Taiwan | Holotype (WSP) (Ju et al. 2005, Hsieh et al. 2009) | EF025592 | EF025607 |
| Rosellinia lamprostoma | none | BCRC34152 | Taiwan | Ju & Hsieh 89112602 (HAST) (Hsieh et al. 2009) | EF025589 | EF025604 |
| Rosellinia necatrix | none | BCRC34153 | Taiwan | Ju & Hsieh 89062904 (HAST) (Hsieh et al. 2009) | EF025588 | EF025603 |
| Stilbohypoxylon elaeicola | none | BCRC34154 | French Guiana | Huhndorf 928 (Rogers and Ju 1997, as S. moelleri; Hsieh et al. 2009) | EF025601 | EF025616 |
| Stilbohypoxylon quisquiliarum | none | BCRC34155 | French Guiana | Huhndorf 940 (Rogers & Ju 1997) (Hsieh et al. 2009) | EF025590 | EF025605 |
| Stilbohypoxylon quisquiliarum | none | BCRC34156 | Taiwan | Ju & Hsieh 89091608 (HAST) (Hsieh et al. 2009) | EF025591 | EF025606 |
| Theissenia (Ootheca) cinerea | none | BCRC34157 | Taiwan | Holotype (HAST) (Ju et al. 2003) | EF025598 | EF025613 |
| Theissenia (Ootheca) rogersii | none | BCRC34158 | Taiwan | Holotype (HAST) (Ju et al. 2007) | EF025597 | EF025612 |
| Whalleya microplaca | none | BCRC34159 | Taiwan | Ju & Hsieh 91111215 (HAST) (Hsieh et al. 2009) | EF025599 | EF025614 |
| Xylaria bambusicola | none | BCRC34102 | Taiwan | Holotype (WSP) (Ju & Rogers 1999, Hsieh et al. 2009)) | AY951873 | AY951762 |
| Xylaria venosula | none | BCRC34160 | USA | Hawaii, Ju & Hsieh 94080508 (HAST) (Hsieh et al. 2009) | EF025602 | EF025617 |
SEM and HPLC
Scanning electron microscopy (SEM) was carried out using a conventional procedure as described in Stadler et al. (2002). Analytical HPLC of stromatal methanol extracts was performed using the standardized method, comprising diode array detection as described by Hellwig et al. (2005) and mass spectrometric detection in the positive and negative electrospray mode, using a comprehensive library of reference compounds (Bitzer et al. 2007). The HPLC reference library included, among numerous other pure natural products, lepraric acid (Læssøe et al. 2010) and various metabolites of the Xylariaceae as authentic standards, allowing for their unambiguous detection in crude extracts by comparison of their retention times, diode array spectra and mass spectra.
RESULTS AND DISCUSSION
Phylogenetic analysis
Fifty-four strains were used in the analysis, 17 of which were Thai material sequenced in this study. From the 17 strains, eight represented isolations from Hypoxylon comedens s.l., five strains from Xylaria, and one strain each from Annulohypoxylon, Biscogniauxia, Hypoxylon, and Kretzschmaria. The remaining 38 sequences across Xylariaceae used were taken from GenBank. Two species ancestral to Xylariales, Cryphonectria macrospora and Amphilogia, were used as outgroup taxa. All 17 strains were sequenced for the α-actin and the β-tubulin gene (Table 1) for comparison with the data in Ju et al. (2007). After initially examining individual trees for α-actin (247 parsimony-informative characters; CI = 0.390, RI = 0.650, RC = 0.253, HI = 0.610) and β-tubulin (1165 parsimony-informative characters; CI = 0.384, RI = 0.588, RC = 0.226, HI = 0.616) these were combined based on the similar topologies of the individual trees.
Of the 2528 characters in the combined alignment, 1412 characters were parsimony informative. Maximum parsimony analyses yielded four most parsimonious trees that had similar topologies except for the terminal branches. One of the four trees generated from maximum parsimony (CI = 0.383, RI = 0.597, RC = 0.229, HI = 0.617) is shown in Fig. 1. The result of MrModeltest selected the General Time Reversible (GTR) model with proportion in invariable sites (I) and gamma distribution (G) (GTR+I+G; Tamura & Nei 1993). This model was then used in MrBayes. Four MCMC chains were run in MrBayes for 2 M generations, sampling every 100 generations. From the 20 K trees obtained the first 2 K trees were discarded as ‘burn-in’. The remaining 18 K trees were pooled and a consensus tree was created. The Bayesian analysis gave a similar result to the maximum parsimony analysis and the PP results were shown as numbers below the branches of the tree (Fig. 1).
Fig. 1.
Phylogenetic relationships of Theissenia and Durotheca species within Xylariaceae generated from a combined β-tubulin and α-actin gene dataset. Numbers above each branch represent bootstrap values and those below the branch are posterior probabilities.
The eleven H. comedens s.l. and Theissenia sequences all fall in a well-supported clade without other elements. The relationship to other groups within Xylariaceae is less clear, but the clade is definitely outside the subfamily ‘Xylarioideae’ that constitute a highly supported cluster. Moreover, the H. comedens s.l. material falls in two well-supported sister clades, with one clade further divided in two based on a limited number of substitutions. T. pyrenocrata falls in a well-supported, rather distant basal position. Theissenia rogersii constitutes a sister group to the combined H. comedens s.l. and T. cinerea clades. On molecular phylogenetic evidence in combination with morphological evidence, we thus recognize two genera and four species in the H. comedens/Theissenia complex, with a further possible separation in the H. comedens complex.
Scanning electron microscopy (SEM)
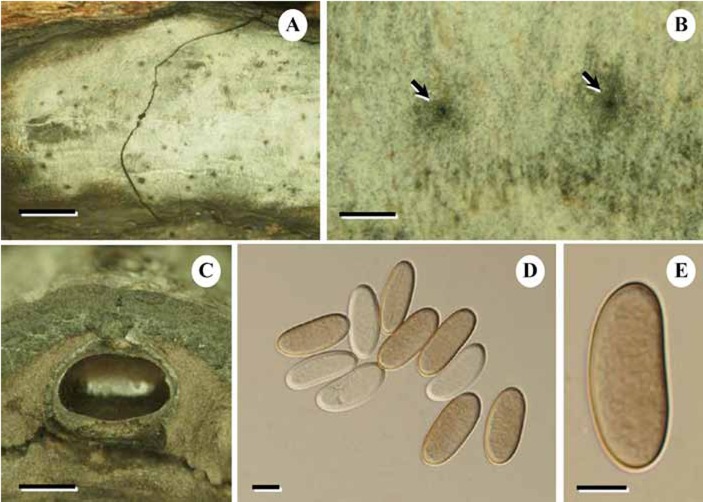
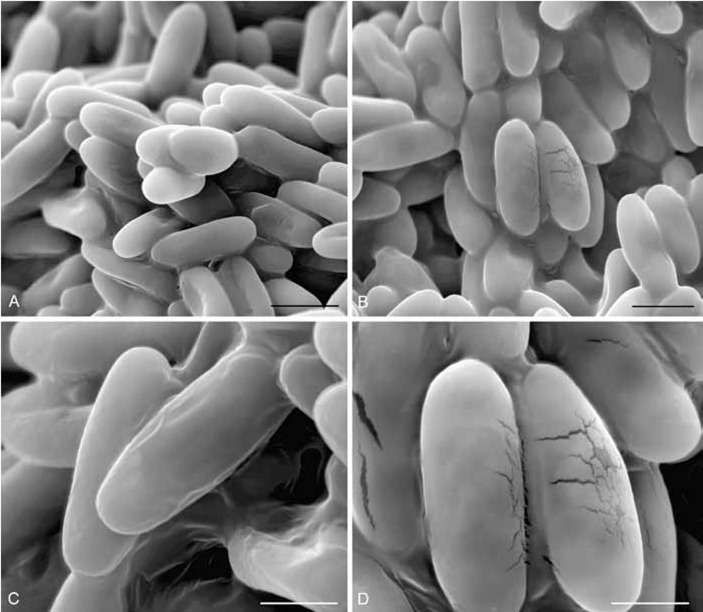
The images obtained from spores of Thai Hypoxylon comedens material (Fig. 5) confirm the results in Læssøe et al. (1989), i.e. that the spores are completely smooth as they also appear to be in KOH mounts at 1200× (including the type material). The possible germ slit observed in that study could not be found in these better preserved ascospores, Since definite germ sites have not been observed by LM, we can conclude that this species lacks obvious germ sites. Occasionally, some of the ascospores, when mounted in 10 % KOH, appeared to have germ slit-like features, but this may have been due to an artefact created by creasing or folding when the spores collapse (or longitudinal ruptures may occur before germination). In any case, even meticulous observations of ascospores of these materials have not revealed a germ slit, as generally observed in many other xylariaceous species. Nevertheless, an ascospore showing a very faint germ slit-like structure, that could just be about to germinate, was observed in the type of D. depressa (see below)
HPLC analyses
Young as well as mature stromata of specimen BBH 15200, identified as Hypoyxlon comedens, were studied for extrolites by HPLC. As previously reported for Biscogniauxia species and various other members of ‘Xylarioideae’ (Stadler et al. 2001, Stadler & Hellwig 2005), none of the characteristic compounds usually encountered in species of Hypoxylon and its immediate allies were found. Not even very young stromata contained binaphthalene BNT, azaphilones, cytochalasins, and other products that occur in various species of Daldinia and Hypoxylon, as well as in their cleistocarpous relatives, Pyrenomyxa, Phylacia, and Rhopalostroma (Stadler et al. 2004, 2005; 2010a,b). These results, in conjunction with morphological features and that the species is devoid of visible and extractable stromatal pigments, indicate that the closest chemical relationships of H. comedens within Xylariaceae are with Biscogniauxia and Camillea, and that the above-mentioned taxa containing pigments are more distantly related. It should, nevertheless, be noted that there are no strong chemotaxonomical syndromes connecting H. comedens with species of Biscogniauxia and Camillea. We observed some minor components, especially in the young, freshly collected stromata, that were apparently absent in the latter genera as well, but could not be safely assigned to any of the known Xylariaceae metabolites. Recently, Læssøe et al. (2010) examined some peculiar taxa assigned to Xylariaceae that deviate from the mainstream of the family in having conspicuous green or blue stromatal surfaces, i.e. H. aeruginosum and representatives of the genus Chlorostroma. Aside from specimens growing fungicolously on stromata of Hypoxylon, the above taxa did not yield any known compounds of the `Hypoxyloideae′, but the substituted chromone, lepraric acid, which had hitherto only been found in lichenized ascomycetes, and derivatives thereof, were detected as major stromatal components of both Chlorostroma and H. aeruginosum. Due to these findings, our attention was directed toward such compounds, also in other Xylariaceae that we examined during our ongoing study using the well-established HPLC profiling technique. One of the recently collected specimens of H. comedens (XY01706/BBH26963) yielded particularly high amounts of yellowish pigments in KOH and was also studied by HPLC. Surprisingly, it yielded lepraric acid, too. The amounts of the compound present in the stromata were estimated to be at least ten times lower in H. comedens than in Chlorostroma and H. aeruginosum, but its identity with lepraric acid (or an isomer thereof) was established by matching DAD and mass spectra. The compound was not detectable at all in mature stromata, suggesting that its biosynthesis only occurs in the initial stages of stromatal formation and ceases as the stromata become mature and carbonaceous. However, traces of lepraric acid were also found in BBH15200 (the one studied by SEM). No molecular data and no DNA suitable for PCE were so far obtained for Chlorostroma and H. aeruginosum. The ascospores of these fungi do not easily germinate, and their stromata are very rarely observed and collected. Therefore, the significance of these findings remains to be confirmed by means of molecular phylogeny, and by studying their conidiogenous structures (in those species that produce them).
MORPHOLOGY AND TAXONOMY
Durotheca Læssøe, Srikitikulchai, Luangsa-ard & M. Stadler, gen. nov.
MycoBank MB803610
Etymology: Indicative of the highly carbonized perithecia without surrounding tissue, easily seen on the underside of detached stromata.
Description: Stromata more or less erumpent through bark or wood, bipartite in nature, initially covered in white pruina, highly carbonaceous including encasement of large, globose to cylindrical perithecia without or with an indistinct basal columella; crust without extractable pigments or with yellow pigmentation. Paraphyses filiform, attenuating towards the apex, distantly septate, without obvious contents. Asci more or less clavate, thin-walled without apical apparatus, deliquescent early, the spores in a tight cluster. Ascospores moderate to very thick-walled, pale to medium brown at maturity, ellipsoid-oblong to allantoid, with or without a germ-slit. Asexual morph, where known nodulisporium-like. Lignicolous, terrestrial.
Type species: Durotheca depressa Læssøe & Srikitikulchai 2013.
Durotheca depressa Læssøe & Srikitikulchai, sp. nov.
MycoBank MB803611
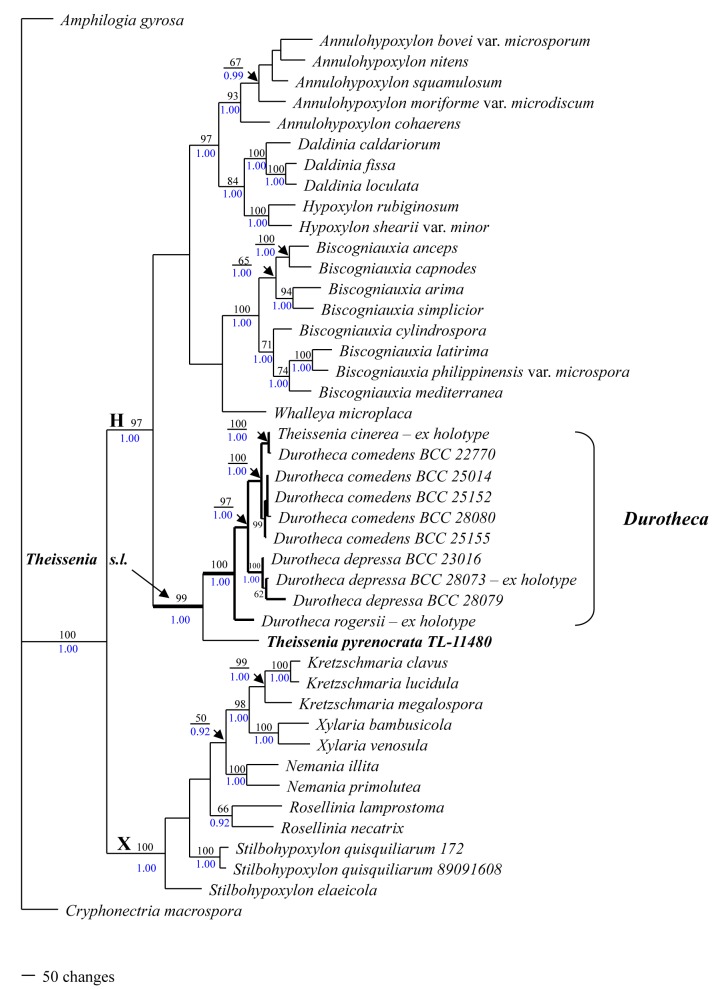
Fig. 2.
Durotheca depressa (BCC23016). A. Stromata. B. Stromata with ostioles, arrow = ostioles. C. Deep ostiole: arrow = deep ostiole. D. Perithecia. E–G. Ascospore: arrow = germination slit. Bars: A = 1.5 mm; B = 0.7 mm; C = 0.5 mm; D = 1.0 mm; E–G = 6.0 μm.
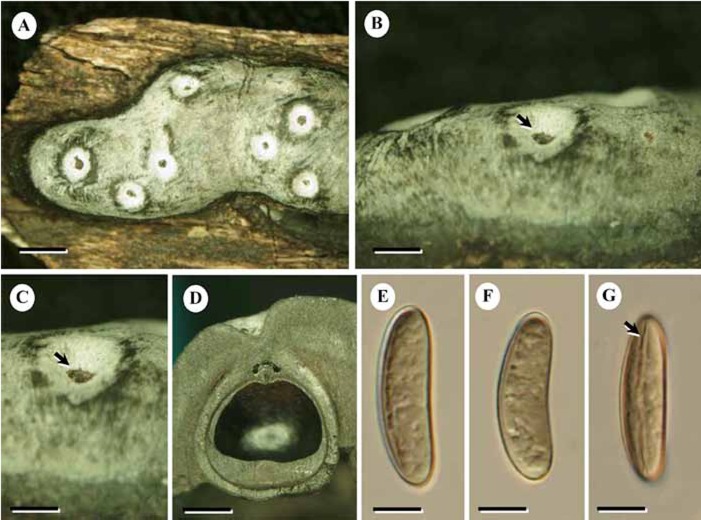
Fig. 3.
Durotheca depressa (BCC28073). A. Stromata. B. Stromata with ostioles: arrow = ostioles. C. Deep ostiole: arrow= deep ostiole. D. Perithecia. E–H. Ascospores. Bars: A = 1.5 mm; B = 1.0 mm; C = 0.5 mm; D = 1.0 mm; E–H = 5.0 μm.
Etymology: Based on the deeply seated ostioles.
Diagnosis: Differs from Durotheca comedens in narrow stromata with deeply seated ostioles in crater like depressions.
Type: Thailand: Prov. Chiang Mai: Doi Inthanon National Park, Pun Churee study trail, on indet. angiosperm wood, 9 May 2006, P. Srikitikulchai XY00402 (BBH 18222 – holotype; BBC 23016 – culture ex-holotype).
Description: Stromata seen from above very narrow and often undulating, effused-pulvinate, with beveled margins, 0.5–6 cm long, 0.5–2 cm broad, up to 2 mm thick; at first chalky white creamy owing to the presence of a thin pruina with mature surface light grey, plane with umbilicate ostioles deep in crater-like depressions; crust highly carbonaceous extending downward to encase each perithecium; tissue between perithecia scarce, fibrous and soft, extending into interstices of overlying carbonaceous stroma; tissue beneath perithecia thin and fibrous to almost absent. Perithecia globose-ovoid, 2.5 mm diam, with conspicuous basal columella. Paraphyses not observed. Asci deliquescing, not observed. Ascospores light brown to brown (absent pigmentation in KOH), unicellular, oblong to allantoid in side view, smooth, wall thick, (19−)20–24(−26) × 8–11 μm (av. 21.9 × 9.3 μm, n = 10), with straight, inconspicuous germ slit spore length; perispore non-dehiscent in 10 % KOH.
Cultures: No conidiogenous structures were produced in cultures derived from the type and paratypes. The morphology of the cultures resembled those of Durotheca rogersii (Ju et al. 2003). The mycelia were initially whitish, melanising with age, the reverse attained a brownish colour with age and even the bramble like structures described by Ju et al. (2003) were evident in ageing cultures.
Host: Unidentified, huge log (possibly Dipterocarpaceae).
Distribution: Only known from a single site at the Doi Inthanon Mountain in northern Thailand.
Additional material (from the same log): Thailand: Prov. Chiang Mai: Doi Inthanon National Park, Pun Churee study trail, alt. 1679 m, on indet. angiosperm wood, 28 May 2006, P. Srikitikulchai XY00619 (BBH 19737, BCC 28073).
Notes: This new species has been repeatedly collected from the same very big log and is so far only known from this material at mid-elevation at the Doi Inthanon Mountain. Durotheca comedens has been collected on an adjacent trail so the two species co-exist at this site. Already in the field the peculiar features of D. depressa were noted (i.e. the narrow, undulating stromata with ostioles in deep depressions), and the phylogenetic data corroborates the distinction. We have chosen this taxon as type of Durotheca since we consider it of value to have DNA sequences from type material.
Durotheca comedens (Ces.) Læssøe & Srikitikulchai, comb. nov.
MycoBank MB803613
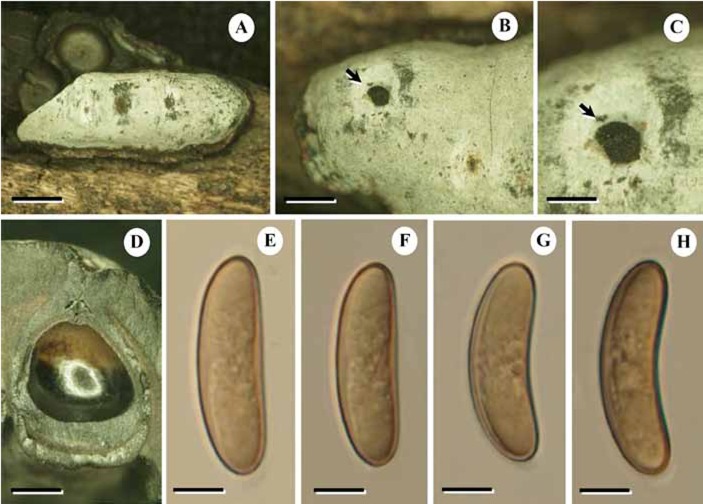
Fig. 4.
Durotheca comedens BBH15200. A. Stromata. B. Stromata with ostioles, arrow: ostioles. C. Perithecia. D–E. Ascospores. Bars: A = 1.0 mm; B = 0.7 mm; C = 1.0 mm; D = 5.0 μm; E = 5.0 μm.
Fig. 5.
SEM of mature ascospores of O. comedens (BBH 15200). A, B. 2000x. C, D. 5000x. Fig. 5D shows disruptions on the surface of the spores in the center, and the spore to the left has been ripped open. Bars: A = 10.0 μm; B = 10.0 μm; C = 5.0 μm; D = 5.0 μm.
Basionym: Hypoxylon comedens Ces., Atti Accad. Sci. fis. mat. Napoli 8: 19 (1879).
Synonyms: Nummularia comedens (Ces.) Cooke, Grevillea 11 (no. 60): 126 (1883)
Nummulariola comedens (Ces.) P. Martin, Jl S. Afr. Bot. 35: 318 (1969) [basionym as “Nummularia comedens Ces.”].
Type: Malaysia: Borneo: Sarawak, [O. Beccari] 218 (K - several, incl. one presumed to be in Cesati handwriting –isotypes).
Theissenia cinerea Y.M. Ju et al., Mycologia 95: 111 (2003).
Type: Taiwan: Pingtung Co., Heng-chun, Ken-ting, on wood stump, 16 July 2001, Hsieh & Ju 90071615 (HAST – holotype).
Description: Stromata erumpent, often sunk rather deep in the decorticated wood, possibly reflecting repeated sporulation in the same position, from above rather variable in outline, from almost circular to very elongate and somewhat irregular, applanate or slightly convex, with abrupt, bevelled dark margins; initially covered by a black, outer, dehiscent layer, exposing a thin white, fairly fugacious, pruinose layer on top of the black, highly carbonised upper stroma, with ostioles in dark pits. Perithecia globose, highly carbonized, densely packed below the crust with hardly any surrounding tissue, or sometimes with a small amount of fibrous tissue below some of them; the base of the perithecia convex to concave, evident in remnants left on the wood when stromata are dislodged, 2.5 mm in diam. Paraphyses as in Hypoxylon/Xylaria (not filled with lipids as in most Camillea species) with distant septation and gradually tapering upwards.
Asci clavate-pedicellate, very early deliquescent and thin-walled, 8-spored. Ascospores in a densely packed cluster, young spores appearing very thick-walled with a central granular part, older spores with pale yellow-brown walls (in water, olivaceous in KOH), suballantoid to allantoid in side view and oblong in front view, few to many guttulate, 15–23(−26) x (5.5−)6–9(−11) μm (av. 16.1–22.4 x 5.9–7 μm, n = 90).
Conidiogenous structures: None were found in cultures from the Thai material, but Ju et al. (2003) reported a nodulisporium-like state with long, slender conidia in the Taiwanese material of T. cinerea.
Host: Stromata appear to be restricted to large, fallen, decorticated dicotyledoneous logs in “wet tropical forests” at low altitudes. No specific hosts have been identified but members of Dipterocarpaceae are likely candidates.
Distribution: Apparently restricted to South-East Asia, where it appears to be widespread, although unrecorded in many places within the region. Tai (1979) and Zhuang (2002) reported it from China. Ju & Rogers (1999) did not include this species in their detailed account of Taiwanese Xylariaceae. Material reported as Hypoxylon cf. comedens from Mexico by San Martín González & Rogers (1993) should be re-evaluated as it may represent a species of Theissenia.
Specimens examined: China: Yunnan:, Xichou, 18 May 1959, Wang Quing-zhi 194 (HMAS 33628(S). – Malaysia: Malay Peninsula: State of Perak, Maxwell’s Hill, alt. 3800 ft, on dead trunk, growing where the bark is removed, 23 Mar 1924, J. H. Burkill 13193 (K); Borneo: Sarawak, Gunong Mulu NP, 4th Division, Baram District, between Melinau Gorge and ca 2 km upstream on S side of Sungei Melinau, alt. ca 150-170 m, on leaning decorticate trunk in alluvial forest, no date, B. J. Coppins 5168 (E, C); Sabah, Danum Valley, Field Centre, West Trail/Rhino Ridge Trail, on old, decorticated trunk in lowland dipterocarp rain forest, alt. 150-200 m, 3 Feb 1999, T. Læssøe & J. Omar, TL-6118 [old, weathered material] (C, UMS). – Thailand: [* indicates that specimens are included in the phylogenetic analysis, Fig. 1] Prov. Chaiyaphum: Phu Khiao Wildlife Sanctuary, Ban Chak Kha, on indet. wood, 23 Oct 2007, P. Srikitikulchai XY00854 (BBH 22419). Prov. Chiang Mai: Doi Inthanon National Park, Pa Mek – Pa Tonnam Lamthan Nature Trail, on indet. angiosperm wood, 26 Nov 2008, P. Srikitikulchai XY001464 (BBH 25163 (BCC 34524)). Prov. Kamphaeng Phet: Khlong Lan National Park, indet. dicot. wood, 7 Nov 2007, P. Srikitikulchai XY00771, XY00834, -835, -836 & -837 (BBH 22341 (BCC 28439), 22400 (BCC 28746), 22401 (BCC 28747), 22402 (BCC 28748) [22401 and 22402 from the same log], 22403 (BCC 28749)). Prov. Nakhon Si Thammarat: Khao Nan National Park, Sunantha Waterfall, on indet. dicot. wood, 20 Feb 2007, P. Srikitikulchai XY00513 & XY00531* (BBH 25875 (BCC 25014), 25876 (BCC 25152)); ibid., Pa Pra nature trail, on indet. dicot. wood, 30 Oct 2008, P. Srikitikulchai XY01412 (BBH 25466 (BCC 33654)). Prov. Phattalung: Khao Puu-Khao Ya National Park, a mixture of young white stromata and very old grey to black stromata, on blackened, very thick, hard-wooded, but very wet, decorticated, dicot log in calcareous lowland, wet evergreen forest, 22 Feb 2006, T. Læssøe & P. Srikitikulchai XY00212 (BBH 15200 (BCC 21319)); ibid., Khao Ban That Wildlife Sanctuary, Khao Chet Yot, 19 Mar 2007, P. Srikitikulchai XY00866 (BBH 22430,(BCC 28891)). Prov. Phet Buri: Kaeng Krachan National Park, Paneontung, indet. dicot. wood, 28 June 2007, P. Srikitikulchai XY00638* (BBH 19755 (BCC 28080)); ibid., Ban Krang, indet. dicot. wood, 26 July 2007, P. Srikitikulchai XY00535 (BBH19738). Prov. Phitsanulok,: Phu Hin Rong Kla National Park, on indet. dicot. wood, 8 Sep 2006, P. Srikitikulchai XY00290*, XY00291 (BBH 1816 & 18117). Prov. Surat Thani. Khao Sok National Park, Sanyang Roi nature trail, 14 Oct 2008, on indet. dicot. wood, P. Srikitikulchai XY01415 (BBH 25469 (BCC 33657)). Prov. Trang: Khao Ban That Wildlife Sanctuary, indet. wood, 17 Mar 2007, P. Srikitikulchai XY00534*, XY00535 (BBH 25877 (BCC 25155), BBH 25878 (BCC 25156)).
Notes: We found the material from a wide geographical area to be morphologically identical, including the isotypes of Hypoxylon comedens, the type of Theissenia cinerea, and material from peninsular Malaysia, the Bornean part of Malaysia, Thailand, and China. The only deviating material is described above as Durotheca depressa. Ju et al. (2003) stated that the perispores of D. comedens (as T. cinerea) ascospores were dehiscent in 10 % KOH and they also provide a picture to support this statement. In all the material we studied of D. comedens, and likewise in the type of T. cinerea, dehiscence was neither observed upon addition of KOH to water mounts, nor when perithecial contents were mounted directly in 10 % KOH. A similar phenomenon, i.e. the occurrence of material with dehiscent and indehiscent perispores in different specimens assigned to the same species, was also attributed to other Xylariaceae in the past (cf. Daldinia fissa, Ju et al. 1997). As we did not find any other deviating criterion to distinguish D. comedens and T. cinerea, we regard these species names as synonyms.
Durotheca rogersii (Y.M. Ju & H.M. Hsieh) Srikitikulchai & Læssøe, comb. nov.
MycoBank MB803632
Basionym: Theissenia rogersii Y.M. Ju & H.M. Hsieh, in Ju et al., Mycologia 99: 613 (2007).
Notes: Durotheca rogersii is placed here based on the description and molecular data provided by Ju et al. (2007), which leave no doubt on the affinities of this species to Durotheca.
Notes on Theissenia pyrenocrata
Theissen (1908) described this new species, from southern Brazil (Rio Grande do Sul), as Ustulina pyrenocrata. Maublanc (1914) coined a new generic name for it, and reported it from further north in São Paulo State, and Ju et al. (2003) even from northeastern Brazil. Dennis (1964) and Ju et al. (2003) reported it from Africa (`Zaire′, now Democratic Republic of Congo), while Miller (1961) and Ju et al. (2003) confirmed its presence in Sri Lanka based on the type of Nummularia porosa that Dennis (1964) considered a likely additional Theissenia species with smaller spores. Here we add a record from western South America that agrees in all morphological characters with those reported in the cited references. It grew on a very large, unidentified hardwood log in black water, inundated lowland rainforest in the eastern part of Ecuador, and was used for sequence analysis. Despite the wide distribution, very few records are known of this rather conspicuous and characteristic species. Ju et al. (2003) discovered the striate-furrowed nature of the ascospores that had been overlooked by previous workers.
Specimens examined: Ecuador: Prov. Orellana: along small black water tributary to Río Tiputini near Tiputini Field Station, alt. 190-270 m, 16 July 2004, T. Læssøe, J.H. Petersen, A. Alsgård Jensen TL-11480 (C, QCNE).
Notes on Theissenia eurima
Theissenia eurima was described from Brazil by Ju et al. (2003). We accept this taxon in Theissenia at present, since it apparently produces an asexual morph equivalent to that of T. pyrenocrata, both taxa occur in South America, and since we have no other morphological or molecular data to suggest another position. There are several other examples of xylariaceous genera that encompass species with and without germ slits, Nemania being an obvious well-known example, aside from the new genus established here.
Key to taxa in the Theissenia-Durotheca clade (Fig. 1)
1 Ascospores striate, almost cylindrical with one side slightly flattened, without germ slit ...........................T. pyrenocrata
Ascospores smooth, with or without germ slit, ellipsoid to slightly allantoid .....................................................................2
2 (1) Ascospores with short germ slit; known from Amazonian Brazil ..........................................................................T. eurima
Ascospores with very faint germ slit, or without obvious germ slit; known from SE Asia Durotheca...................................3
3 (2) Ascospores broadly ellipsoid, wall very thick, 25–36 μm long; perithecia cylindrical ........................................ D. rogersii
Ascospores ellipsoid-cylindrical to allantoid, usually less than 25 μm long, wall moderately thickened; perithecia subglobose ............................................................................................................................................................................... 4
4 (3) Stromata with variable outline, not narrowly linear, ostioles in shallow depressions ...................................D. comedens
Stromata more or less linear with ostioles in crater-like depressions ............................................................ D. depressa
DISCUSSION
As already noted, the clade with Theissenia pyrenocrata and taxa placed in Durotheca here (Fig. 1) has a rather unresolved position within Xylariaceae. However, the clade has very limited affinities to the ‘Xylarioideae’ subclade, whilst it is difficult to speculate on affinities to the ‘Hypoxyloideae’, but such relationships cannot be ruled out at present. The highly carbonized, very thick, and layered ascomatal wall more or less seated directly on the substrate is a common feature of all currently recognised members of Theissenia and Durotheca. The two genera are also separated on several stromatal characters and, possibly, in the type of asexual morph. The molecular phylogenetic data show two very distinct groups with T. pyrenocrata in a well-supported basal position. Further phylogenetic analyses would probably benefit from an expanded taxon sampling and from the inclusion of other genes. Ju et al. (2007), in their analysis, placed Theissenia s.lat. within the `Hypoxyloideae′ in a clade containing species with a bipartite stromatal development.
The chemotaxonomic data so far available on these fungi are rudimentary at best, since the cultures have not been studied for metabolites, and the surprising detection of lepraric acids in young stromata of some representatives merely provides a hint as to their possible affinities to other Xylariaceae. Due to the study by Bitzer et al. (2008), a rather comprehensive overview of chemical traits in cultures of the hypoxyloid clade have become available, but Biscogniauxia, Camillea, as well as the xylarioid Xylariaceae, were underrepresented in this work. In addition, no cultures and no molecular data on Hypoxylon aeruginosum and Chlorostroma species have so far been available, and, therefore, it is at present difficult to assess whether the production of lepraric acid derivatives has a common history in the taxa with green and blue coloured stromatal surfaces and Durotheca. Interestingly, these substituted chromones seem to be very rare even in lichenised ascomycetes, where they have hitherto only been found in members of the rather distantly related genera Lepraria and Roccella, aside from the above mentioned Xylariaceae (cf. Huneck & Yoshimura 1996, Læssøe et al. 2009). Notably the previous studies on lichen chemotaxonomy mostly relied on thin layer chromatography, rather than the much more sophisticated and sensitive HPLC-MS technique, and the data presented here are actually based on studies of several thousands of Xylariaceae specimens. On the other hand, the absence of the typical pigments of Hypoxylon and allies in all the above taxa, as well as in Biscogniauxia and Camillea may support the molecular phylogeny. It is too early to draw final conclusions on the affinities of basal groups of Xylariaceae as inferred from molecular phylogenetic studies. However, studies based on rDNA and other DNA sequence data (Pelaez et al. 2008, Tang et al. 2009) have also suggested that Biscogniauxia and Camillea might be basal to both the xylarioid and the hypoxyloid lineages. Unfortunately, these studies, as well as other phylogenetic work cited above, have dealt with different isolates, different genes, and, to some extent, even different species concepts. These issues mean that results cannot be directly compared. Possibly, Durotheca, Theissenia, and even Chlorostroma and H. aeruginosum may represent hitherto unknown lineages that separated quite early from the ancestors of mainstream Xylariaceae. The availability of living cultures of Durotheca and Theissenia will now facilitate further testing of such hypotheses.
Acknowledgments
This study was supported by Bioresources Research Network (BRN), the TRF/BIOTEC special programme for biodiversity research and training (BRT), and the National Center for Genetic Engineering and Biotechnology (BIOTEC) who provided working facilities. We wish to thank Nigel Hywel-Jones, Somsak Sivichai, and Sumalee Supothina for their help with logistics and with the project, including the handling of cultures. The aid of Klaus Ide (BIS, Leverkusen, Germany) with performing SEM is gratefully acknowledged, as is the help of the curators at C, E, HMAS, K, and QCNE. Brian J. Coppins (Edinburgh) is thanked for supplying material from Sarawak. Research in Ecuador was sponsored by Danish government grant RUF 91056, and co-operation with the Herbario Nacional (Quito) is duly recognized.
Footnotes
1This subfamily name does not appear to have been validly published, but is nevertheless widely used.
REFERENCES
- Bitzer J, Köpcke B, Stadler M, Hellwig V, Ju YM, Seip S, Henkel T. (2007) Accelerated dereplication of natural products, supported by reference libraries. Chimia 51: 332–338 [Google Scholar]
- Bitzer J, Læssøe T, Fournier J, Kummer V, Decock C, Tichy HV, Piepenbring M, Peršoh D, Stadler M. (2008) Affinities of Phylacia and the daldinioid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycological Research 112: 251–270 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Cesati V. (1879) Mycetum in itinere Borneensi lectorum a cl. Od. Beccari. Atti della Real Accademia delle Scienze Fisiche e Matematiche, Napoli 8: 1–28 [Google Scholar]
- Dennis RWG. (1964) Further records of Congo Xylariacae. Bulletin de Jardin Botanique de l’État, Bruxelles 34: 231–241 [Google Scholar]
- Hall T. (2004) BioEdit. Version 6.0.7. Durham: Department of Microbiology, North Carolina State University [Google Scholar]
- Hellwig V, Ju YM, Rogers JD, Fournier J, Stadler M. (2005) Hypomiltin, a novel azaphilone from Hypoxylon hypomiltum, and chemotypes in Hypoxylon sect. Hypoxylon as inferred from analytical HPLC profiling. Mycological Progress 4: 39–54 [Google Scholar]
- Hsieh HM, Lin CR, Fang MJ, Rogers JD, Fournier J, Lechat C, Ju YM. (2010) Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Molecular Phylogenetics and Evolution 54: 957–969 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist FR. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Biometrics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Huneck S, Yoshimura I. (1996) Identification of Lichen Substances. Berlin: Springer [Google Scholar]
- Ju YM, Hsieh H-M, Ho M-C, Szu D-H, Fang M-J. (2007) Theissenia rogersii sp.nov. and phylogenetic position of Theissenia. Mycologia 99: 612–621 [DOI] [PubMed] [Google Scholar]
- Ju YM, Rogers JD. (1994) Kretzschmariella culmorum (Cooke) comb. nov. and note on some other monocot-inhabiting xylariaceous fungi. Mycotaxon 51: 241–255 [Google Scholar]
- Ju YM, Rogers JD. (1999) The Xylariaceae of Taiwan (excluding Anthostomella). Mycotaxon 73: 343–440 [Google Scholar]
- Ju YM, Rogers JD, Hsieh HM. (2003) The genus Theissenia: T. pyrenocrata, T. cinerea sp. nov., and T. eurima sp. nov. Mycologia 95: 109–116 [DOI] [PubMed] [Google Scholar]
- Ju YM, Rogers JD, Hsieh HM. (2004) New Hypoxylon species and notes on some names associated with or related to Hypoxylon. Mycologia 96: 154–161 [PubMed] [Google Scholar]
- Ju YM, Rogers JD, San Martín F, Granmo A. (1998) The genus Biscogniauxia. Mycotaxon 66: 1–98 [Google Scholar]
- Ju YM, Rogers JD. (2001) New and interesting Biscogniauxia taxa, with a key to the world species. Mycological Research 105: 1123–1133 [Google Scholar]
- Læssøe T. (1994) Index ascomycetum 1. Xylariaceae. Systema Ascomycetorum 13: 43–112 [Google Scholar]
- Læssøe T, Srikitikulchai P, Fournier J, Köpcke B, Stadler M. (2010) Lepraric acid derivatives as chemotaxonomic markers in Hypoxylon aeruginosum Chlorostroma subcubispora and C. cyaninum, sp. nov. Fungal Biology 114: 481–489 [DOI] [PubMed] [Google Scholar]
- Laessøe T, Rogers JD, Whalley AJS. (1989) Camillea, Jongiella and light-spored species of Hypoxylon. Mycological Research 93: 121–155 [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. (2007) ClustalW and ClustalX version 2. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Mackill DJ, Bonman JM. (1995) Classifying japonica rice cultivars with RAPD markers. Crop Science 35: 889–894 [Google Scholar]
- Martin P. (1969) Studies in the Xylariaceae. VI Daldinia, Nummulariola and their allies. Journal of South African Botany 35: 267–320 [Google Scholar]
- Maublanc A. (1914) L’Ustulina pyrenocrata Theissen, type du genre nouveau Theissenia. Bulletin de la Societé mycologique de France 30: 48–53 [Google Scholar]
- Miller JH. (1961) A Monograph of the World Species of Hypoxylon. Athens, GA:University of Georgia Press [Google Scholar]
- Nylander JAA. (2004) MrModeltest 2.2. Uppsala: Distributed by the author, Evolutionary Biology Centre, Uppsala University [Google Scholar]
- O’ Donnell K, Cigelnik E. (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116 [DOI] [PubMed] [Google Scholar]
- Peláez F, González V, Platas G, Sánchez-Ballesteros J, Rubio V. (2008) Molecular phylogenetic studies within the family Xylariaceae based on ribosomal DNA sequences. Fungal Diversity 31: 111–134 [Google Scholar]
- Rogers JD, Ju YM. (1998) The genus Kretzschmaria. Mycotaxon 68: 345–393 [Google Scholar]
- San Martín González F, Rogers JD. (1993) Biscogniauxia and Camillea in Mexico. Mycotaxon 47: 229–258 [Google Scholar]
- Stadler M, Wollweber H, Mühlbauer A, Asakawa Y, Hashimoto T, Rogers JD, Ju YM, Wetzstein H-G, Tichy H-V. (2001) Molecular chemotaxonomy of Daldinia and other Xylariaceae. Mycological Research 105: 1191–1205 [Google Scholar]
- Stadler M, Baumgartner M, Ide K, Popp A, Wollweber H. (2002) Importance of ascospore ornamentation in the taxonomy of Daldinia. Mycological Progress 1: 31–42 [Google Scholar]
- Stadler M, Hellwig V. (2005) Chemotaxonomy of the Xylariaceae and remarkable bioactive compounds from Xylariales and their associated asexual stages. Recent Research and Development in Phytochemistry 9: 41–93 [Google Scholar]
- Stadler M, Ju Y-M, Rogers JD. (2004) Chemotaxonomy of Entonaema, Rhopalostroma and other Xylariaceae. Mycological Research 108: 239–256 [DOI] [PubMed] [Google Scholar]
- Stadler M, Læssøe T, Vasilyeva L. (2005) The genus Pyrenomyxa and its affinities to other cleistocarpous Hypoxyloideae as inferred from morphological and chemical traits. Mycologia 97: 1129–1139 [DOI] [PubMed] [Google Scholar]
- Stadler M, Fournier J, Gardt S, Peršoh D. (2010a) The phylogenetic position of Rhopalostroma as inferred from a polythetic approach. Persoonia 25: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M, Fournier J, Læssøe T, Chlebicki A, Lechat C, Flessa F, Rambold G, Peršoh D. (2010b) Chemotaxonomic and phylogenetic studies of Thamnomyces (Xylariaceae). Mycoscience 51: 189–207 [Google Scholar]
- Swofford DL. (2003) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0b10. Sunderland, MA: Sinauer Associates [Google Scholar]
- Tai FL. (1979) Sylloge fungorum sinicorum. Science Press, Peking [Google Scholar]
- Tamura K, Nei M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biolology and Evolution 10: 512–526 [DOI] [PubMed] [Google Scholar]
- Tang AMC, Jeewon R, Hyde KD. (2009) A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Diversity 34: 155–153 [Google Scholar]
- Theissen F. (1908) Novitates riograndenses. Annales Mycologici 16: 341–352 [Google Scholar]
- Thiers B. (2010) (continuously updated) Index Herbariorum: A global directory of public herbaria and associated staff. New York: New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/ih [Google Scholar]
- Wiens JJ. (1998) Combining data sets with different phylogenetic histories. Systematic Biology 47: 568–581 [DOI] [PubMed] [Google Scholar]
- Zhuang WY. (2001) Higher Fungi of Tropical China. Beijing: Mycotaxon [Google Scholar]