Abstract
Gelatinomyces siamensis gen. sp. nov., incertae sedis within Leotiomycetes, the Siamese jelly-ball, is described. The fungus was collected from bamboo culms and branches in Nam Nao National Park, Phetchabun, Thailand. It presents as a ping-pong ball-sized and golf ball-like gelatinous ascostroma. The asci have numerous ascospores, are thick-walled, and arise on discoid apothecia which are aggregated and clustered to form the spherical gelatinous structures. An hyphomycete asexual morph is morphologically somewhat phialophora-like, and produces red pigments. On the basis of phylogenetic analysis based on rRNA, SSU, and LSU gene sequences, the lineage is closest to Collophora rubra. However, ITS sequences place the fungus on a well-separated branch from that fungus, and the morphological and ecological differences exclude it from Collophora.
Keywords: Bambusa, Bambusicolous fungi, Collophora, Gelatinous ascostroma, Kao-niew ling, Siamese jelly-ball, Molecular phylogeny, Polyspored asci, Red pigments
INTRODUCTION
Five specimens of a rarely encountered fungus were collected by N. S. from twigs of a bamboo (“Bong”; Bambusa nutans) in Nam-Nao National Park, Thailand, in August and September 2009–2011. The local name, “Siamese jelly-ball” or “kao-niew ling”, recalls the dark, golf ball-like and gelatinous ascostromata, and it is claimed to be edible. It has only been found on bamboo, and was not seen on any other plants in the area. It occurs at 390–840 m, where the average temperature is generally less than at lower and flatter localities.
Numerous bambusicolous fungi have been reported, with the number of fungal genera reportedly greater in the tropical regions than other regions due to the higher number of bamboo species. More than 630 species of fungi are known from bamboo, most of which are ascomycetes; Eriksson & Yue (1998) discuss 587 names of pyrenomycetes described on bamboo, and approximately 200 species occur in south-east Asia (Hyde et al. 2002). However, few produce distinctive to sometimes very large ascostroma similar to those seen in the Thai fungus. Daldinia bambusicola (Ju et al. 1997) has a black, smooth surface, and relatively smaller ascostromata. Engleromyces goetzei produces very large ascostromata, up to 4.5 kg in weight, and E. sinensis is also considerably larger than Gelatinomyces; these two species appear to be confined to particular bamboo species normally found on very high mountains (Whalley et al. 2010). The hypocrealean fungi, Ascopolyporus philodendrus (Bischoff et al. 2005), Moelleriella gaertneriana (Chaverri et al. 2008), and Mycomalus bambusinus (Bischoff & White 2003), produce rather pale, smooth-walled or brain-like ascostromata and are probably associated with insects. Munkia martyris, Neomunkia sydowii and Ustilaginoidea virens are other hypocrealean fungi in the tribe Ustilaginoideae producing large asexual stromata on bamboo twigs but their relationships have not yet been resolved (Bischoff et al. 2005). In addition, Shiraia bambusicola (Dothideomycetes, Pleosporomycetidae) produces spectacular pinkish orange ascostromata (Liu et al. 2012). All taxa mentioned above have perithecoid or flask-shaped ascomata, 8-spored asci, ascospores that are not to several septate, may or may not have interascal filaments, and occur on living leaves or branches.
Since the Siamese jelly-ball fungus is distinct from any previously scientifically named fungus, it is described as a monotypic new genus here. It does, however, have some affiliation to Collophora, but molecular evidence supports its separation.
MATERIALS AND METHODS
Collecting and field sites
Five specimens were collected from Bambusa nutans, along creeks in Nam Nao National Park, Thailand, at an altitude of 390–840 m. The first specimen was found behind the Nam Nao Tourist Service area in late September 2009, and the next four specimens were from bamboo along the main road in August and September 2011. Bamboo branches or culms with specimens were cut off from the main culms, wrapped in newspaper and brought back to the Plant Pathology Laboratory, Faculty of Agriculture, Khon Kaen University, for isolation into pure culture. Dried reference specimens and living cultures have been deposited at the Khon Kaen University Culture Collection (KKUK), at Biotec Culture Collection (GESIASCO), CBS and the Royal Botanic Gardens Kew (K; GESI).
Isolation, spore discharge, and germination
Two isolation techniques were employed to obtain pure cultures: tissue transplanting from parts of ascomata, and ascospores forced to eject directly from asci by exposing a piece of ascomata to incandescent light, Phillips 220V, 15W, for a few minutes. Ejected ascospores were collected on PDA plates or in sterile Petri dishes. The ejected ascospores on PDA plates were allowed to germinate directly to form colonies, while those in the empty Petri dishes were diluted in sterilized water and subsequently plated out on PDA plates to obtain single ascospore isolates. All white colonies forming within 3–4 d, with diffusible red pigment in the agar on the reverse side of these colonies were then selected and maintained for further study.
Morphological investigation
Fresh gelatinous ascostromata and thin sections of ascomata embedded in paraffin wax were examined by light microscopy (Olympus Model BX51 and DP21-LPT) equipped with anOlympus Nomarski Slider for Transmitted Light U-DICT (Olympus Model U-DICT) to record the detailed morphology of the sexual and asexual morphs. Slide cultures of representative isolates, from stroma, single ascus, and single ascospore isolations, were also examined microscopically for the development of asexual structures and for the production of crystals of insoluble pigment. Structures were mounted in water, and 30 measurements (at 1 000 ×) made of each feature. The 5th and 95th percentiles were defined for all measurements, and the extremes are given in parentheses, including the value of the mean ± SD and L/W ratio (Damm et al. 2008, Gramaje et al. 2012). To investigate discrete conidiomata production, water agar culture plates with sterile pine needles were placed under conditions defined by Gramaje et al. (2012) for 4–5 wk. Ascospores and conidia were suspended in distilled water then air dried on cellulose acetate filter paper (Sartorius Stedim Biotech, Bohemia, NY) for scanning electron microscopy. The samples were sputter-coated with a film of gold using a Polaron Range SC7620 sputter coater and examined under a LEO 145OVP scanning electron microscope.
DNA extraction, PCR amplification, DNA sequencing, and phylogenetic analysis
The genomic DNA of representative strains isolated from single asci and single ascospores was extracted from active growing mycelia on PDA plates using a cetyltrimethylammonium bromide (CTAB) protocol (Jeewon et al. 2004, Cai et al. 2006). The whole and partial sequences from three different regions of the rDNA molecules; the ribosomal small subunit (SSU), large subunit (LSU), and internal transcribed spacer (ITS), characterised by different rates of evolution, were amplified by PCR using primers having sequences and target regions shown in Table 1. Whole sequences of SSU were cloned using pGEM-T Easy Vector (Promega, Promega Corporation, Madison, WI) and Escherichia coli DH5α as a host. The amplification conditions were performed in a 50 μL reaction volume as follows: 1 × PCR buffer (Invitrogen™ Life Technologies, Foster, CA), 0.2 mM each dNTP, 0.3 μM of each primer, 1.5 mM MgCl2, 0.8 units Tag DNA Polymerase (Invitrogen™ Life Technologies), and 100 ng DNA. PCR parameters for all the regions were as follows: initial denaturation at 94 °C for 3 min, 30 cycles of 94 °C for 1 min, 52 °C for 50 s, and 72 °C for 1 min, and final extension of 72 °C for 10 min. The PCR amplified products were examined by electrophoresis using 1 % agarose gel containing ethidium bromide (0.5 μzzg mL). The separated PCR products were then observed under short wavelength UV light. DNA sequencing was performed using the primers as mentioned above in an Applied Biosystems 3730 DNA Analyser at Macrogen Inc (#60-24, Gasan-dang, Geumchen-gu, Seoul, Korea). Since we could not assign or differentiate the fungus to known taxa, we used SSU, LSU and ITS for sequence comparisons and in BLASTn searches (www.ncbi.nlm.nih.gov). The rDNA sequences of the new fungus have been deposited in GenBank under accession numbers JX219377 and JX219378 (SSU), JX219381 and JX219382 (LSU), and JX219379 and JX219380 (ITS regions including 5.8S rDNA) for isolates KKUK1 and KKUK2, respectively.
Table 1.PCR primers used for obtaining DNA sequences of the Gelatinomyces siamensis.
| Name | Sequence (5’-3’) | Target regiona | Reference |
|---|---|---|---|
| NS1 | GTAGTCATATGCTTGTCTC | SSU 20-38 | White et al. (1990) |
| NS4 | CTTCCGTCAATTCCTTTAAG | SSU 1150-1131 | White et al. (1990) |
| SR8R | GAACCAGGACTTTTACCTT | SSU 732-749 | Vilgalys & Hester (1990) |
| NS8 | TCCGCAGGTTCACCTACGGA | SSU 1788-1768 | White et al. (1990) |
| ITS4 | TCCTCCGCTTATTGATATGC | Internal transcribed spacer (ITS) regions LSU 60-41 | White et al. (1990) |
| ITS5 | GGAAGTAAAAGTCGTAACAAGG | SSU 1744-1763 | White et al. (1990) |
| NL1 | GCATATCAATAAGCGGAGGAAAAG | Domain of large subunit (LSU) rDNA | O’Donnell (1993) |
| NL4 | GGTCCGTGTTTCAAGACGG | D1/D2 domain of LSU rDNA | O’Donnell (1993) |
a Saccharomyces cerevisiae numbering.
To construct the phylogenetic tree, the analysis was modified from Greif et al. (2007), instead of using two taxa, Orbilia auricolor and Scutellinia scutellata as outgroup, only O. auricolor was employed. The sequence data of the Siamese Jelly-ball were aligned by ClustalX2 with sequences of 60 species retrieved from GenBank (www.ncbi.nlm.nih.gov) representing different classes of ascomycete fungi, including Arthoniomycetes, Dothideomycetes, Eurotiomycetes, Le-canoromycetes, Leotiomycetes, Lichinomycetes, and Sor-dariomycetes, where both SSU and LSU sequences data were available (Table 2), and manually edited by MEGA 5.05. A data set comprising all known species of Collophora with available ITS sequences (Table 3) was used for comparison and the outgroups for this dataset were Neobulgaria pura and Leotia lubrica. A maximum parsimony analysis was conducted using PAUP v. 4.0b10 (Swofford 1998). A heuristic search was performed using parsimony as the optimality criterion. Gaps were treated as missing data. Starting trees were obtained at random via stepwise addition with tree-bisection-reconnection as the branch-swapping algorithm, and with the MulTrees option in effect. After 100 stepwise additional sequences were completed, confidence in the branches of the resulting trees was evaluated by bootstrap analysis (Felsenstein 1985) using 1000 replicates. The resultant tree was visualized using PAUP v. 4.0b10 (Swofford 1998). Additionally, Bayesian analysis using MrBayes version 3.2.1 which approximates posterior probabilities of clades using a Markov chain-Monte Carlo (MCMC) method (Huelsenbeck & Ronquist 2001) were performed. Four chains for a total of 2 500 000 generations for SSU and LSU datasets and 600 000 generations for ITS dataset with phylogenetic trees sampled every 100 generations were applied to all searches. The general time-reversible model with invariant sites and gamma distribution (GTR + I + Γ) were used. Scores in Baysian analyses were estimated as posterior probabilities calculated from the posterior distribution of trees excluding 25 % burn-in trees (Huelsenbeck & Rannala 2004). Nodes obtained from both analysis were considered well supported by bootstrap values greater than or equal to 70 % and posterior probabilities greater than or equal to 0.95 (Spatafora et al. 2007).
Table 2.Fungal taxa used for phylogenetic analysis with GenBank accession numbers for small subunit (SSU) and large subunit (LSU) sequences, including their main characteristics.
| Ingroup | GenBank accession no. (SSU, LSU) | Origin (substrate, country) | Main characteristics | Reference |
|---|---|---|---|---|
| Class Sordariomycetes | ||||
| Cainia graminis | AF431948, AF431949 | Sesleria albicans, France | Stromatic perithecial, unitunicate with pore, saprophytic, plant parasitic or endophytic | Lumbsch et al. (2005) |
| Chaetomium globosum | AB048285, AY346272 | Indoor environment, Germany | Huhndorf et al. (2004), Okane et al. (2001) | |
| Diatrype disciformis | DQ471012, DQ470964 | Decayed wood, Netherlands | Spatafora et al. (2006) | |
| Hypocrea americana | AY544693, AY544649 | Fomitopsis pinicola, USA | Lutzoni et al. (2004) | |
| Sordaria fimicola | AY545724, AY545728 | Dung, Canada | Cai et al. (2006) | |
| Xylaria acuta | AY544719, AY544676 | Decayed wood, USA | Rogers (1984) | |
| Xylaria hypoxylon | AY544692, AY544648 | Downed rotting wood, USA | Spatafora et al. (2006) | |
| Class Leotiomycetes | ||||
| Botryotinia fuckeliana | AY544695, AY544651 | Apothecial or cleistothecial, unitunicate and inoperculate, saprophytic, plant parasitic, some species known only anamorphic i.e. Collophora | Hirschhauser & Frohlich (2007) | |
| Bulgaria inquinans | DQ471008, DQ470960 | Germany | Spatafora et al. (2006) | |
| Collophora rubra | GQ154628, GQ154608 | Wood necrosis close to pruning wound, South Africa | Damm et al. (2010) | |
| Crinula calciiformis | AY544729, AY544680 | Lutzoni et al. (2004) | ||
| Monilinia fructicola | AY544724, AY544683 | Fruit, USA | Fulton & Brown (1997) | |
| Neofabraea malicorticis | AY544706, AY544662 | Apples, USA | Lutzoni et al. (2004) | |
| Pezicula carpinea | DQ471016, DQ470967 | Carpinus caroliniana, Canada | Spatafora et al. (2006) | |
| Potebniamyces pyri | DQ470997, DQ470949 | Cankered bark, USA | Spatafora et al. (2006) | |
| Class Lecanoromycetes | ||||
| Diploschistes thunbergianus | AF274112, AF274095 | Australia | Apothecial, unitunicate, rostrate asci, mostly lichenized | Lumbsch et al. (2005) |
| Lobaria scrobiculata | AY584679, AY584655 | USA | Lutzoni et al. (2004) | |
| Trapella placodioides | AF119500, AF274103 | Wall, UK | Lumbsch et al. (2005) | |
| Class Lichinomycetes | ||||
| Lempholemma polyanthes | AY548805, AF356691 | USA | Apothecial, fissitunicate, lichenized | Lutzoni et al. (2004) |
| Peltula auriculata | DQ832332, DQ832330 | Miadlikowska et al. (2006) | ||
| Peltula umbilicata | DQ782887, AF356689 | Miadlikowska et al. (2006) | ||
| Class Eurotiomycetes | ||||
| Eremascus albus | M83258, AY004345 | Dried fruit, UK | Cleistothecial, non-fissitunicate, saprophytic or plant parasitic | Berbee & Taylor (1992) |
| Eurotium rubrum | U00970, AY004346 | Lumbsch et al. (2005a) | ||
| Penicillium expansum | DQ912698, AF003359 | Fruit, USA | Seifert & Louis-Seize (2000) | |
| Exophiala dermatitidis | DQ823107, DQ823100 | Human, USA | James et al. (2006) | |
| Glyphium elatum | AF346419, AF346420 | Salix, USA | Lindemuth et al. (2001) | |
| Ramichloridium anceps | DQ823109, DQ823102 | Soil under Thuja plicata, Canada | James et al. (2006) | |
| Class Dothideomycetes | ||||
| Order Botryosphaeriales | ||||
| Botryosphaeria ribis | DQ678000, DQ678053 | Ribes, USA | Pseudothecial, fissitunicate asci, saprophytic or plant parasitic | Schoch et al. (2006) |
| Botryosphaeria stevensii | DQ678012, DQ678064 | Fraxinus excelsior, Netherlands | Schoch et al. (2006) | |
| Guignardia bidwellii | DQ678034, DQ678085 | Parthenocissus tricuspidata | Schoch et al. (2006) | |
| Order Capnodiales | ||||
| Catenulostroma abetis | DQ678040, DQ678092 | Abies, Germany | Pseudothecial, fissitunicate asci, saprophytic or plant parasitic | Schoch et al. (2006) |
| Cercospora beticola | DQ678039, DQ678091 | Beta vulgaris, Italy | Schoch et al. (2006) | |
| Microxyphium citri | AY016340, AY004337 | Fruit of Citrus sinensis, Spain | Lumbsch et al. (2005) | |
| Mycosphaerella punctiformis | DQ471017, DQ470968 | Dead fallen leaves of Quercus robur, Netherlands | Spatafora et al. (2006) | |
| Scorias spongiosa | DQ678024, DQ678075 | Aphid | Schoch et al. (2006) | |
| Order Dothideales | ||||
| Aureobasidium pullulans | DQ471004, DQ470956 | Fruit of Vitis vinifera, France | Pseudothecial, fissitunicate asci, pseudoparaphyses absent, mainly saprophytic | Spatafora et al. (2006) |
| Delphinella strobiligena | AY016341, AY016358 | Cone of Pinus halepensis, Greece | Lumbsch & Lindemuth (2001) | |
| Discosphaerina fagi | AY016342, AY016359 | Leaf of Populus, UK | Lumbsch & Lindemuth (2001) | |
| Dothidea ribesia | AY016343, AY016360 | Cult of Ribes, Switzerland | Lumbsch et al. (2005) | |
| Stylodothis puccinioides | AY016353, AY004342 | Viburnum lantana, Switzerland | Lumbsch et al. (2005) | |
| Order Myriangiales | ||||
| Cladosporium cladosporioides | DQ678004, DQ678057 | Leaf of Arundo, England | Pseudothecial, fissitunicate globose asci, non-ostiolar, saprophytic or plant parasitic | Schoch et al. (2006) |
| Davidiella tassiana | DQ678022, DQ678074 | Human skin, Netherlands | Schoch et al. (2006) | |
| Elsinoe centrolobi | DQ678041, DQ678094 | Centrolobium robustum, Brazil | Schoch et al. (2006) | |
| Myriangium duriaei | AY016347, DQ678059 | Chrysomphalus aonidium, Argentina | Lumbsch & Lindemuth (2001) | |
| Order Pleosporales | ||||
| Arthopyrenia salicis | AY538333, AY538339 | Bark of Salix, Netherlands | Perithecoid pseudothecial, ostiolar, non-lichenized or lichenized with fissitunicate asci and pseudoparaphyses present | Lumbsch et al. (2005) |
| Cucurbitaria elongata | DQ678009, DQ678061 | Cytisus sessilifolius, France | Schoch et al. (2006) | |
| Dendrographa leucophaea | AY548803, AY548810 | Lutzoni et al. (2004) | ||
| Lecanactis abietina | AY548805, AY548812 | Lutzoni et al. (2004) | ||
| Neotestudina rosatii | DQ384069, DQ384107 | Seed of Cuminum cyminum imported from India, Japan | Kruys et al. (2006) | |
| Pleospora herbarum | DQ247812, DQ247804 | Leaf of Medicago sativa, India | Schoch et al. (2006) | |
| Setosphaeria monoceras | AY016352, AY016368 | Lumbsch & Lindemuth (2001) | ||
| Trematosphaeria heterospora | AY016354, AY016369 | Iris, Switzerland | Lumbsch et al. (2005) | |
| Westerdykella cylindrica | AY016355, AY004343 | Cow dung, Kenya | Lumbsch et al. (2005) | |
| Order Incertae sedis, Family Tubeufiaceae | ||||
| Helicomyces lilliputeus | AY856942, AY856899 | Rotten dicotyledonous wood, USA | Pseudothecial, fissitunicate | Tsui & Berbee (2006) |
| Helicomyces roseus | DQ678032, DQ678083 | Submerged bark, Switzerland | Schoch et al. (2006) | |
| Tubeufia cerea | AY856947, AY856903 | Tsui & Berbee (2006) | ||
| Class Arthoniomycetes | ||||
| Arthonia dispersa | AY571379, AY571381 | Syringa vulgaris, Sweden | Fissitunicate, mostly lichenized | Lumbsch et al. (2005) |
| Dendrographa leucophaea | AY548803, AY548810 | Lutzoni et al. (2004) | ||
| Lecanactis abietina | AY548805, AY548812 | Lutzoni et al. (2004) | ||
| Unknown | ||||
| Gelatinomyces siamensis | Bambusa nutans, Thailand | Apothecial, aggregated, embedded in gelatinous ball | This study | |
| Isolate KKUK1 | JX219377, JX219381 | |||
| Isolate KKUK2 | JX219378, JX219382 | |||
| Outgroup Class Orbiliomycetes | ||||
| Orbilia auricolor | DQ471001, DQ470953 | Soil, UK | Apothecial, non-fissitunicate | Spatafora et al. (2006) |
Table 3.Fungal taxa in the Collophora species, Leotiomycetes used for phylogenetic analysis with GenBank accession numbers for ITS sequences, including their main characteristics.
| Species | GenBank accession no. (ITS) | Origin (substrate, country) | Main characteristics | Reference |
|---|---|---|---|---|
| Order Incertae sedis, Family Incertae sedis | ||||
| Collophora africana | GQ154570 | Prunus salicina, South Africa | Hyphae carry short necks or mere collarettes that release conidia; discrete conidiomata present | Damm et al. (2010) |
| C. capensis | GQ154571 | Prunus salicina, South Africa | As above | Damm et al. (2010) |
| GQ154572 | ||||
| GQ154573 | ||||
| GQ154574 | ||||
| C. hispanica | JN808840 | Prunus dulcis, Spain | As above | Gramaje et al. (2012) |
| JN808841 | ||||
| JN808842 | ||||
| C. paarla | GQ154586 | Prunus salicina, South Africa | As above | Damm et al. (2010) |
| C. pallida | GQ154578 | Prunus salicina, South Africa | As above | Damm et al. (2010) |
| GQ154580 | ||||
| GQ154582 | ||||
| GQ154584 | ||||
| C. rubra | GQ154562 | Prunus salicina, South Africa | As above | Damm et al. (2010) |
| GQ154564 | ||||
| GQ154566 | ||||
| GQ154568 | ||||
| Gelatinomyces siamensis | Bambusa nutans | Sexual morph present, asexual conidia produced on short and long conidiogenous cells | This study | |
| Isolate KKUK1 | JX219379 | Thailand | ||
| Isolate KKUK2 | JX219380 | |||
| Outgroup-Leotiales | ||||
| Leotia lubrica | GU222296 | |||
| Neobulgaria pura | HM051080 | |||
RESULTS
The total number of characters in the SSU analysis was 2954, including gaps. All characters were given equal weight. The number of constant characters was 1122, and 1039 were parsimony-informative. The maximum parsimony analysis yielded a single tree with 4968 characters. The scores of the tree were as following; Consistency index (CI) = 0.623, Retention index (RI) = 0.468, Rescaled consistency index (RC) = 0.291, and Homoplasy index (HI) = 0.377. In the partial LSU sequence, the total number of characters from the alignment was 644 with gaps; 268 and 299 out of these 644 characters were constant and parsimony-informative, respectively. Only one tree was generated by maximum parsimony analysis from the LSU data set, with CI = 0.338, RI = 0.635, RC = 0.215, and HI = 0.622.
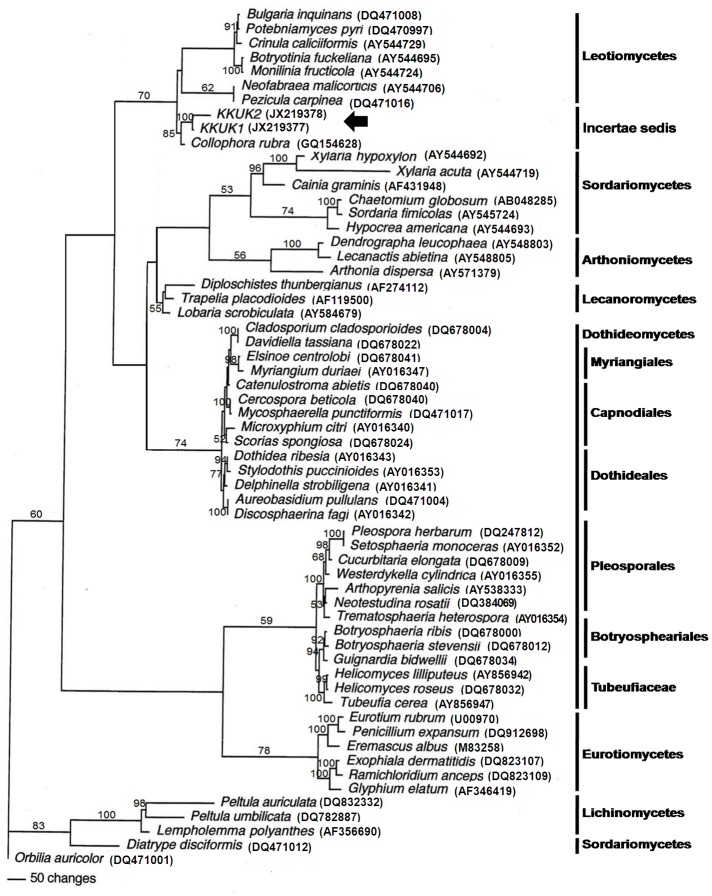
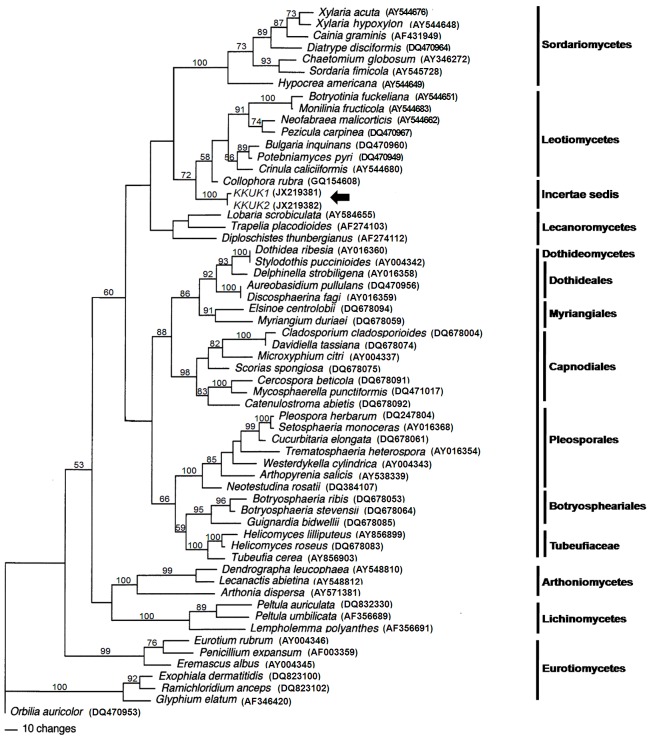
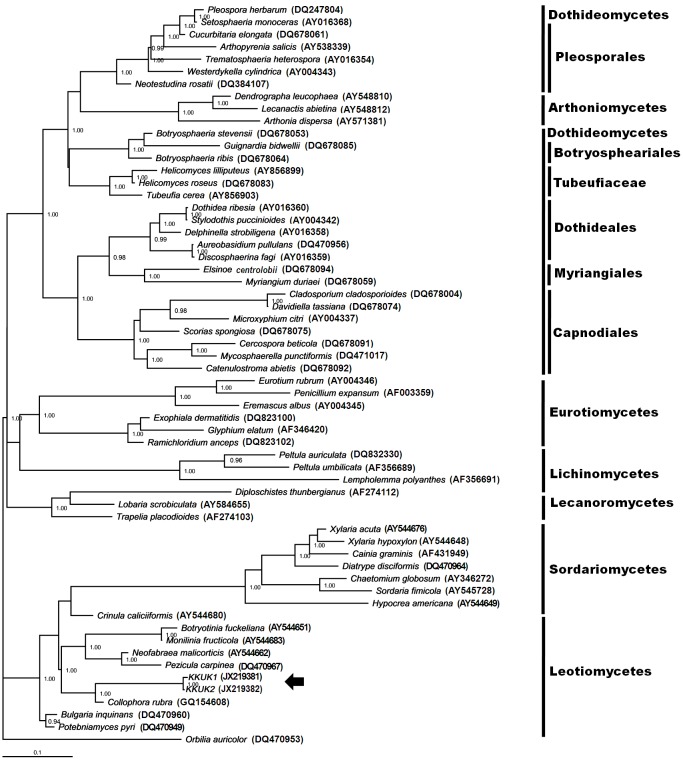
In the SSU tree, only members of one class of fungi grouped in the same clade as isolates KKUK1 and KKUK2. These belonged to Leotiomycetes, with a bootstrap score of 70 (Fig. 1). This suggests that the two isolates KKUK1 and KKUK2 are Leotiomycetes. To ascertain their closest sequenced relatives, the SSU sequences were also compared to those available in GenBank using the standard nucleotide-nucleotide BLAST program. Isolates KKUK1 (JX219377) and KKUK2 (JX219378) had the highest sequence similarity with Collophora rubra (GQ154628) at 97 %. The LSU tree gave a similar result, with the studied isolates KKUK1 and KKUK2 clustered in Leotiomycetes, with a bootstrap score of 72 (Fig. 3). BLASTn searches of SSU and LSU sequences of the isolates confirmed these results.
Fig. 1.
Phylogenetic tree from maximum parsimony analysis based on SSU sequences showing the position of Gelatinomyces siamensis isolates KKUK1&2 (arrow), which is grouped closely in Leotiomycetes. Bootstrap support values > 50 % are shown above branches.
Fig. 3.
Phylogenetic tree from maximum parsimony analysis based on LSU sequences showing the position of Gelatinomyces siamensis isolates KKUK1&2 (arrow), which clusters very close to Leotiomycetes. Bootstrap support values > 50 % are shown above branches.
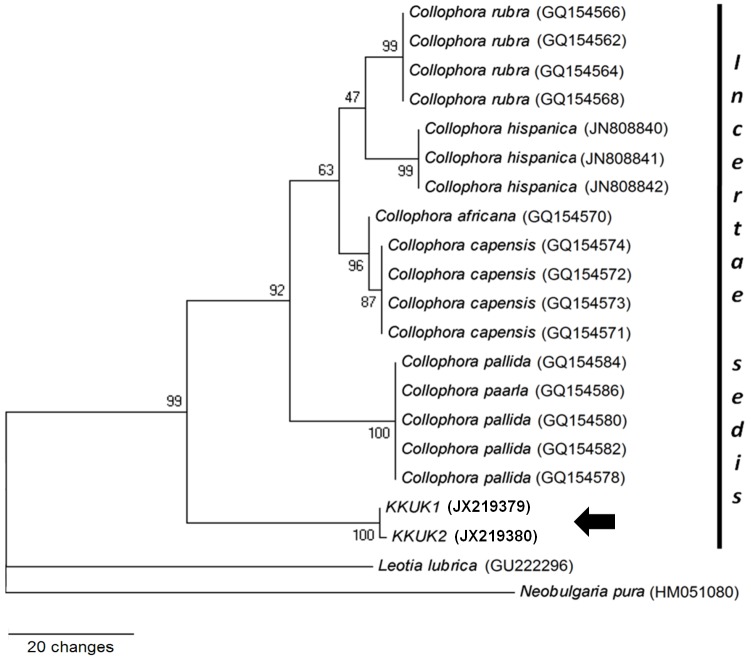
To determine whether the bamboo fungus was a Collophora species or not, an additional ITS tree was constructed; this had 638 characters, of which 380 were constant characters and 100 parsimony informative. The tree was parsimoniously constructed with Neobugaria pura and Leotia lubrica as outgroups (CI = 0.893, RI = 0.886, RC = 0.791, and HI = 0.107). KKUK1 and KKUK2 clustered together (bootstrap score = 100) and were separated from six Collophora species with bootstrap support at 99 (Fig. 5).
Fig. 5.
Phylogenetic tree from maximum parsimony analysis based on ITS sequences showing the position of Gelatinomyces siamensis isolates KKUK1&2 (arrow) which clusters very close to Collophora spp. Bootstrap support values > 50 % are shown above the branches.
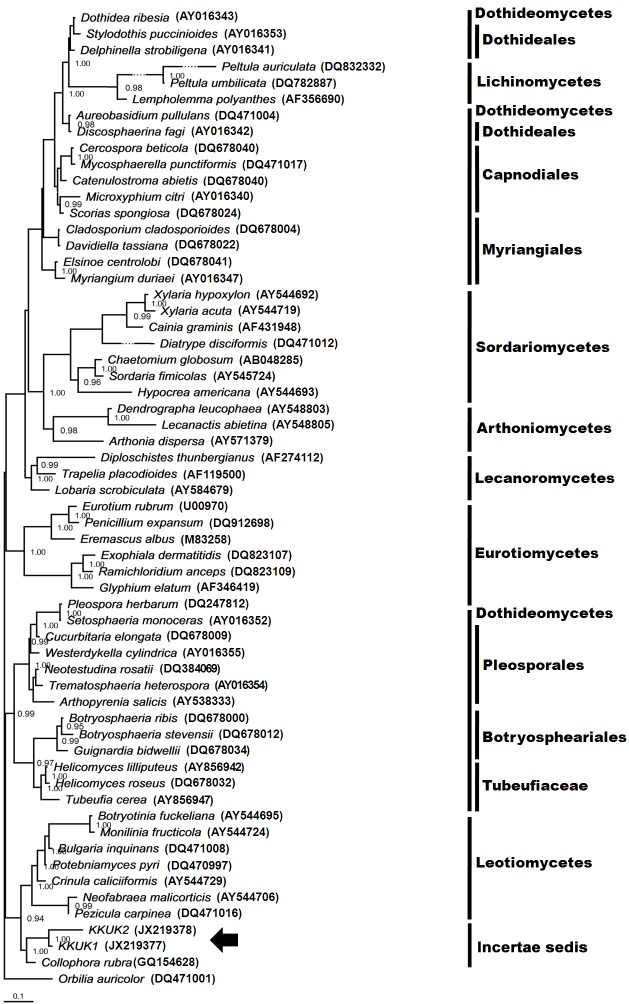
The SSU and LSU trees inferred by Bayesian analysis gave phylogenetic relationship results similar to those employed by maximum parsimony, although the topologies of the trees were different (Figs 2, 4). The KKUK1 and KKUK2 isolates were grouped in Leotiomycetes, with posterior probability values of 1.0. Similarly, the additional tree inferred from ITS information using the same dataset employed in Fig. 5 produced a tree with similar topology and support values which indicated that Collophora species were clustered separately from KKUK1 and KKUK2.
Fig. 2.
Phylogenetic tree obtained from Bayesian analysis inferred from SSU sequences showing phylogenetic relationship among fungal species selected from Ascomycota and Gelatinomyces siamensis isolates KKUK1&2. Posterior probability values ≥ 0.95 yielded from a Bayesian analysis shown at nodes. Gelatinomyces siamensis is grouped in Leotiomycetes (arrow).
Fig. 4.
Phylogenetic tree obtained from Bayesian analysis inferred from LSU sequences showing phylogenetic relationship among fungal species selected from Ascomycota and Gelatinomyces siamensis isolates KKUK1&2. Posterior probability values ≥ 0.95 shown at nodes.
On the basis of the DNA sequence analyses from the SSU, LSU, and ITS regions, and the sexual and asexual characters of the fungus, we conclude that Siamese jelly-ball, found on bamboo in Nam Nao National Park, Thailand, is new to science and represents a previously undescribed genus and species, named Gelatinomyces siamensis here.
TAXONOMY
Gelatinomyces Sanoamuang, Jitjak, Rodtong & Whalley, gen. nov.
MycoBank MB804026
Diagnosis: Ascostromata ball-shaped, ping pong ball-sized, gelatinous, dark coloured when mature, with red pigmentation inside. Ascomata apothecia, aggregated, containing thick-walled multi-spored asci.
Etymology: Recalling the gelatinous nature of the ball shaped ascostromata, and myces = fungus.
Type: Gelatinomyces siamensis N. Sanoamuang et al. 2013.
Description: Stromata present, pale grey to dark coloured, soft gelatinous in texture. Ascomata apothecia, aggregated but well separated, translucent, pale grey, convex or cushion-shaped, ± globose or pulvinate when young, later becoming brown-black to black and discoid, flattened or slightly depressed when mature. Apothecia sessile, the exciple dark and gelatinous, well-developed, ± glabrous. Hymenial layer composed of interascal filaments, asci, and with a gelatinous layer at the surface; interascal tissue poorly developed, composed of simple, branched paraphyses. Asci cylindrical, tapered at the base, without an operculum or any opening characters at the tip, non-amyloid apical ring, multi-spored, persistent. Ascospores minute, hyaline, globose to ovoid shaped with smooth walls.
Colonies slow-growing, white, moist at first then becoming dry with age, lacking aerial mycelium. Conidiophores hyaline, of two types, either with very short conidiogenous cells on hyphal cells, or longer conidiogenous cells arising at branching points where a septum forms. Conidia vary in shape and size at first, aggregated in masses around hyphae on the agar surface, becoming ovoid, minute, and powdery with age. No discrete conidiomata observed on sterilized pine needles on the surface of water agar.
Gelatinomyces siamensis Sanoamuang, Jitjak, Rodtong & Whalley, sp. nov. MycoBank MB804027
Fig. 6.
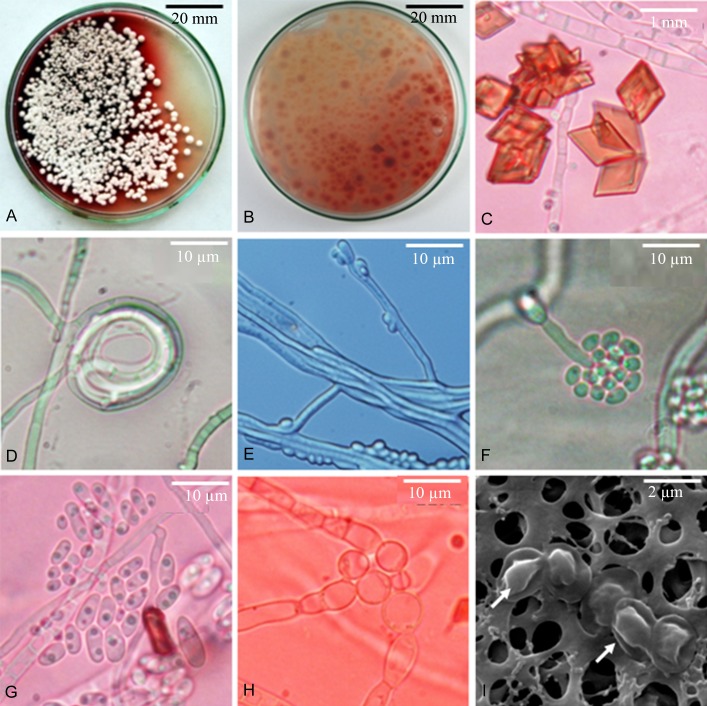
Sexual morph of Gelatinomyces siamensis (A–C, F, G holotype; D, E isotype). A. Ascostromata. B. Apothecia. C. Red pigments accumulated inside ascostroma. D. Ascal arrangement on gelatinous apothecium covered by dark matter. E. Asci and paraphyses. F. Single ascus. G. Ascospores under scanning electron microscope (arrow).
Fig. 7.
Microscopic characteristics of the asexual morph of Gelatinomyces siamensis (ex-holotype). A, B. Fungal colonies on PDA producing red pigments into the media. C. Red crystals generated by mycelia. D. Hyphal coil. E. Hyphal pairing, condia from short conidiogenous cells directly from mycelium. F. Conidia cluster at the apex of the tapered annellides and long, thick-walled, septate conidiophores. G. Conidia with internal inclusions. H. Swollen hypha. I. Dried conidia under scanning electron microscope (arrow).
Diagnosis: Stromata gelatinous, ball shaped, 3-4 cm diam, surface with many discoid ascomata, aggregated but separate, pale greenish to pinkish grey, becoming black when mature, a band of red pigmented in the interior. Asci clavate with a short stipe, unitunicate in structure, multispored. Ascospores tiny, globose to slightly ovoid. Asexual morph Phialosphora-like, conidia produced on very short conidiogenous cells on hyphal cells and also on longer conidiogenous cells. Colonies white, but with a distinctive red pigmented underside, the red pigment diffusing into agar.
Etymology: Named after the country of origin.
Type: Thailand: Phetchabun Province: Nam Nao National Park, on bamboo culms and branches, 11 Sept. 2011, Sanoamuang (KKUK – holotype; KKUK1, 2, 3, 4.... 100 – ex-holotype cultures; Biotec Culture Collection codes: Gesiasco 6, 11, 18, and 19; CBS 135071, 135072, 135073 and 135074; K – Gesi01, 02 and 03 – isotypes).
Description: Stromata, 3–4 cm across, pale grey to brown black, soft and highly gelatinous, inner tissue repeatedly folded, up to golf-ball size when fresh, dark to black, hard and sclerotium-like when dry; 300–560 discoid ascomata aggregated, but separate, embedded in the surface of a single gelatinous stromatic ball. Ascomata apothecia, usually 100–200 μm tall and 340–600 μm diam in surface view, translucent, greyish green, sometimes pale pink, convex or cushion-shaped when young, ± globose or pulvinate, brown-black to black, discoid, flattened or slightly depressed when mature, sessile. Hymenium dark and gelatinous, well-developed, the exciple is smooth, interascal ascal tissues poorly developed, composed of simple, branched paraphyses. Asci (79.5−)84.5–175(−178) × (15−)15.5–31(−31.5) μm, clavate, tapered at the base, without an operculum or any opening structures at the tip, apical ring non-amyloid, multispored, persistent, thick walled but unitunicate in structure, 1–3 μm (av. 1.5 ± 0.5 μm) measured at the central part of asci, slightly thickening towards the tip, penetrating through the gelatinous layer covering asci to forcibly eject ascospores. Ascospores hyaline, globose to ovoid, smooth-walled, 2–2.5 × 1.5–2.5 μm, mean ± SD = 2.2 ± 0.25 × 1.8 ± 0.19 μm, L/W=1.2:1.
Colonies slow-growing, white, moist at first then dry with age, lacking aerial mycelium. Conidiophores hyaline, of two types: (1) Conidiophores reduced to very short conidiogenous cells or conidiogenous pegs arising from hyphal cells, ~1 μm long; and (2) Longer conidiogenous cells, (10−)12.0–46.0(−48) × 2.0–3.0 μm, produced at the branching points where the septum appears. Conidia are single-celled and colourless. Conidia produced on very short conidiogenous cells on hyphal cells, vary in shape and size, (2.5−)3–11.5(−12) x 2.0–5.0 μm, mean ± SD = 6.29 ± 0.32 × 2.79 ± 0.25 μm, L/W=2.3:1, aggregated in masses around the hyphae or around the apex of, annellide-like conidiogenous cells and on the agar surface from conidiogenous pegs. Conidia produced on the longer conidiogenous cells, nearly ovoid, 2.0–4.0 × 2–2.5 μm, mean ± SD = 2.27 ± 0.19 x 2.12 ± 0.16 μm, L/W=1.1:1, similar to conidia obtained from old cultures. Swollen hypha also present.
An unidentified red pigment is always associated with ascostromatic structures and the asexual morph in artificial culture. Patches of red pigment are accumulated inside the ascostroma, visible when cut. The red pigment appears in culture as both diffusible and water insoluble substances. The soluble red pigment stains the medium soon after the establishment of the colony starting under the fungal colonies and covers the whole Petri dish within a week, whereas the insoluble red pigment appears as crystals on the surface of the fungal colony (Fig. 7a–c).
DISCUSSION
Bamboos are the only known habitat for a wide range of fungi, to which can be added Gelatinomyces siamensis. As mentioned in the Introduction, the majority of bamboo fungi reported to produce large stromata are in Sordariomycetes and Dothideomycetes, whereas molecular analyses show that G. siamensis belongs in Leotiomycetes (Figs 1–4), with bootstrap values of 70 and 72 in both SSU and LSU trees, respectively. Further, all the previously reported taxa with large stromata produce perithecioid structures immersed in ascostromatic tissue, whereas G. siamensis produces discoid apothecia on the surface of a gelatinous ascostromatic ball. The discoid apothecia are sessile or very short stalked, and contain thick-walled asci with numerous ascospores originating from the same level in a single layer inside the apothecium. Branched and septate interascal filaments grow between the asci. In the parsimonious tree derived from SSU sequence data, Leotiomycetes diverged before Dothideomycetes and Sordariomycetes.
The ascus type is one of the essential morphological characters used to classify and identify ascomycete fungi. There is a wide range of ascus types, e.g. operculate, poricidal, non-poricidal, deliquescent, fissitunicate and rostrate, based on how ascospores are discharged (Bellemère 1994, Schoch et al. 2009). In Gelatinomyces siamensis, the ascospores are forcibly released when exposed under light through the thick-walled, and apparently multi-layered ascus which is functionally unitunicate. However, there is no evidence that the asci are operculate or porous. Therefore, the G. siamensis ascus is best termed rostrate because when discharging ascospores, the apical part of the ascus is broken to release the spores (Schoch et al. 2009).
In terms of the ascostromatal texture, the gelatinous nature of apothecia is one of the key characteristics mentioned by Wang et al. (2006a, b) to indicate membership of Helotiaceae, and is seen, for example, in Ascocoryne, Ascotremella and Neobulgaria (Seaver 1930, Petersen & Læssøe 2012). An additional feature recognized in this family is an endophytic lifestyle. However, G. siamensis does not appear to be endophytic as the ascostromata are superficially attached to the pole surface and are easily removed without any apparent damage to either the trees or the ascostromata. Gelatinomyces siamensis seems to be associated only with bamboo and its biological role requires further investigation.
Generally, the number of ascospores in an ascus is eight, whereas G. siamensis has numerous ascospores in a single mature ascus. Polyspored asci can originate as a result of one of several different mechanisms: fragmentation of eight originally multiseptate spores, repeated mitotic divisions following meiosis leading to numerous spores being then cut simultaneously from the ascus protoplast, or the direct formation of conidia from ascospores while still in the ascus (Hawksworth 1987, Raju 2002). Polyspory is a diagnostic character in some families and genera in diverse classes and orders of ascomycetes, while in other cases it is phylogenetically informative only at the species level, arising in particular species within genera otherwise comprising 8-spored species. An example from the Leotiomycetes is Thelebolus stercoreus (de Hoog et al. 2005). This feature should, therefore, not be over-emphasized in the recognition of the genus Gelatinomyces, especially as the ontogeny of ascosporogenesis in this fungus has not yet been determined
As the phylogenetic trees obtained from SSU and LSU sequence data and BLASTn results hinted that Collophora rubra was the most closely related species, an ITS dataset containing various ITS sequences from all six known Collophora species was compiled (Table 3). Maximum parsimony and Bayesian analysis of these data confirmed that Gelatinomyces siamensis occupied an isolated position well-separated from the Collophora clade. The separation was supported by bootstrap scores of 99 (Fig. 5). As no sexual morph is currently known in any of the described Collophora species, we speculated that G. siamensis could be a sexual morph of Collophora, but this possibility is excluded by molecular and morphological comparisons.
Further, while bamboos are the natural habitat for G. siamensis, and the ascostromata can easily be detached from the poles, Collophora species live inside peach and almond trees and can be pathogenic. We attempted induction of conidiomata in our cultures, under conditions applied to C. hispanica (Gramaje et al. 2012). Gelatinomyces siamensis did not produce any discrete conidiomata, but only separate tiny conidia instead. In addition, the development of internal conidia inside hyphae, as seen in Collophora, did not occur. On the other hand, conidiogenous cells arose at septal points and swollen hypha were microscopically seen in G. siamensis, whereas Collophora species have not been shown to have either of these characteristics. A comparison of the significant features exhibited by G. siamensis and Collophora species is presented in Tables 4 and 5.
Table 4. Ecological and morphological characteristics of Collophora spp. in comparison to Gelatinomyces siamensis.
| Characteristics |
Species |
|
|---|---|---|
| Gelatinomyces | Collophora | |
| Associated plant | Bamboo species | Prunus spp. and almond |
| Position | Attached to the point where bud breaks, culms or branches | Deep inside the heart of the wood, with heart rot symptom |
| Teleomorph | Apothecia aggregate in a ball-like cluster | Unknown |
| Red pigment crystalline in pure culture | Numerous, parallelogram or rhombus in shape | Absent, not mentioned |
| Conidiogenous pegs, intercalary | Present | Present |
| Conidiogenous cells at the septal point | Present | Absent |
| Swollen hypha as conidial mother cells | Present | Absent |
| Conidia | Various sizes and shapes but turn slightly ovoid in shape and minute in size when age | Consistency in shape and size |
Table 5. Characteristics of Collophora spp. in culture media in comparison to Gelatinomyces siamensis.
| Species | Spore size (μm) | Discrete conidiomata | Pigment | Endo-conidia | Sexual morph |
|---|---|---|---|---|---|
| C. africana | (2.5−)3.5–5.5(−8) × 1–2(−2.5) L/W = 3:1 |
Present | Red | Present | Unknown |
| C. capense | (4−)4.5–6.5(−9) × 1–1.5(−2) L/W = 3.7:1 |
Present | Red | Present | Unknown |
| C. hispanica | (2.5−)3.5–5(−6.5) × (1−)1.5(−2) L/W = 2.9:1 |
Present | Red | Present | Unknown |
| C. paarla | (3−)4–7.5(−11) × (0.5−)1–2(−3) L/W = 4.1:1 |
Present | Yellow, red | Present | Unknown |
| C. pallida | (2.5−)3–5(−7) × 1–1.5(−2) L/W = 3.5:1 |
Present | None | Present | Unknown |
| C. rubra | (3.5−)4–5.5(−8) × 1–2(−3.5) L/W = 3.2:1 |
Present | Red | Present | Unknown |
| G. siamensis | (2.0−)2.1–3.9(−4) × (2−)2–2.5(−2.5) L/W=1.1:1 |
Absent | Red | Absent | Apothecia |
On the grounds of morphological characteristics and molecular phylogeny of the fungus, G. siamensis belongs in phylum Ascomycota, class Leotiomycetes, but cannot be referred to any accepted order at this time; i.e. it has to be treated as incertae sedis within the class. It is conceivable that future molecular data on this and other genera of Leotiomycetes might indicate that a new order is appropriate, but we consider that this would be premature at this time.
Acknowledgments
Grateful acknowledgement is made to the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Holistic Watershed Management Cluster of Khon Kaen University. We are deeply grateful to SUT Research Center for Microbial Cultures for Food and Bioplastics Production, and Narumol Mothong for supporting and assisting with the DNA work. Acknowledgement is extended to Khon Kaen University and the Faculty of Agriculture for providing financial support for manuscript preparation activities.
REFERENCES
- Bellemère A. (1994) Asci and ascospores in ascomycete systematics. In: Ascomycete Systematics: problems and perspectives in the nineties (Hawksworth DL, ed.): 111–126 New York: Plenum Press [Google Scholar]
- Berbee ML, Taylor JW. (1992) Two ascomycete classes based on fruiting-body characters and ribosomal DNA sequences. Molecular Biology and Evolution 9: 278–284 [DOI] [PubMed] [Google Scholar]
- Bischoff JF, Chaverri P, White JF., jr (2005) Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendron sp. nov. Mycologia 97: 710–717 [DOI] [PubMed] [Google Scholar]
- Bischoff JFB, White JF., jr (2003) The plant-infecting clavicipitaleans. In: Clavicipitalean Fungi: evolution, biology, chemistry, biocontrol, and cultural impacts (White JF, jr, Bacon CW, Hywel-Jones NL, Spatafora JW, eds): 119–145 Marcel Dekker: New York [Google Scholar]
- Cai L, Jeewon R, Hyde KD. (2006) Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycological Research 110: 137–150 [DOI] [PubMed] [Google Scholar]
- Chaverri P, Liu M, Hodge KT. (2008) A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the neotropics. Studies in Mycology 60: 1–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Crous PW, Fourie PH. (2008) A fissitunicate ascus mechanism in the Calosphaeriaceae, and novel species of Jattaea and Calosphaeria on Prunus wood. Persoonia 20: 39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Fourie PH, Crous PW. (2010) Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24: 60–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog GS, Gottlich E, Platas G, Genilloud O, Leotta G, van Brummelen J. (2005) Evolution taxonomy and ecology of the genus Thelebolus in Antarctica. Studies in Mycology 51: 33–76 [Google Scholar]
- Eriksson OE, Yue J-Z. (1998) Bambusicolous pyrenomycetes, an annotated checklist. Myconet 1 (2): 25–78 [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Fulton CE, Brown AE. (1997) Use of SSU rDNA group-I intron to distinguish Monilinia fructicola from M. laxa and M. fructigena. FEMS Microbiology Letters 157: 307–312 [DOI] [PubMed] [Google Scholar]
- Gramaje D, Agusti-Brisach C, Pérez-Sierra A, Moralejo E, Olmo D, Mosrt L, Damm U, Armengol J. (2012) Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia 28: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaje D, Mostert L, Armengol J. (2011) Characterization of Cadophora luteo-olivacea and C. melinii isolates obtained from grapevines and environmental samples from grapevine nurseries in Spain. Phytopathology Mediterraneana 50 (Suppl.): 112–126 [Google Scholar]
- Greif MD, Gibas CFC, Tsuneda A, Currah RS. (2007) Ascoma development and phylogeny of an apothecioid dothideomycete, Catinella olivacea. American Journal of Botany 94: 1890–1899 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL. (1987) The evolution and adaptation of sexual reproductive structures in the Ascomycotina.In: Evolutionary Biology of the Fungi (Rayner ADM, Brasier CM, Moore D, eds): 179–189 Cambridge: Cambridge University Press [Google Scholar]
- Hirschhauser S, Frohlich J. (2007) Multiplex PCR for species discrimination of Sclerotiniaceae by novel laccase introns. International Journal of Food Microbiology 118: 151–157 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Rannala B. (2004) Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Systematic Biology 53: 904–913 [DOI] [PubMed] [Google Scholar]
- Hyde KD, Zhou D, Dalisay T. (2002) Bambusicolous fungi: a review. Fungal Diversity 9: 1–14 [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schussler A, Longcore JE, O’Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lucking R, Budel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443: 818–822 [DOI] [PubMed] [Google Scholar]
- Jeewon R, Liew ECY, Hyde KD. (2004) Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity 17: 39–55 [Google Scholar]
- Ju YM, Rogers JD, San Martín F. (1997) A revision of the genus Daldinia. Mycotaxon 61: 243–293 [Google Scholar]
- Kruys A, Eriksson OE, Wedin M. (2006) Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycological Research 110: 527–536 [DOI] [PubMed] [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT. (2001) Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that Loculoascomycetes (Ascomycota) are not monophyletic. Mycological Research 105: 1176–1181 [Google Scholar]
- Liu Y, Liu Z, Wongkaew S. (2012) Developing characteristics and relationships of Shiraia bambusicola with bamboo. Sonklanakarin Journal of Science and Technology 34: 17–22 [Google Scholar]
- Lumbsch HT, Lindemuth R. (2001) Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycological Research 105: 901–908 [Google Scholar]
- Lumbsch HT, Schmitt I, Lindemuth R, Miller A, Mangold A, Fernandez F, Huhndorf S. (2005) Performance of four ribosomal DNA regions to infer higher-level phylogenetic relationships of inoperculate euascomycetes (Leotiomyceta). Molecular Phylogenetics and Evolution 34: 512–524 [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett DS, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lucking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R. (2004) Assembling the fungal tree of life: progress, classification, and evolution of sub-cellular traits. American Journal of Botany 91: 1446–1480 [DOI] [PubMed] [Google Scholar]
- Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lucking R, Hestmark G, Otalora MG, Rauhut A, Budel B, Scheidegger C, Timdal E, Stenroos S, Brodo I, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May P, Schoch CL, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F. (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA and two protein-coding genes. Mycologia 98: 1088–1103 [PubMed] [Google Scholar]
- Minter DW, Peredo HL, Watson AT. (2007) Acrospermum chilense sp. nov. from Chile and the Acrospermales ord. nov. Boletín de la Sociedad Argentina de Botánica 42: 107–112 [Google Scholar]
- O’Donnell K. (1993) Fusarium and its near relatives. In: The Fungal Holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (Reynolds DR, Taylor JW, eds): 225–233 Wallingford: CAB International [Google Scholar]
- Okane I, Nakagiri A, Ito T. (2001) Surculiceries rugispora gen. et sp. nov., a new endophytic mitosporic fungus from leaves of Bruguiera gymnorrhiza. Mycoscience 42: 115–122 [Google Scholar]
- Petersen JH, Læssøe T. (2012) MycoKey 4.0. www.mycokey.com [Google Scholar]
- Raju NB. (2002) Meiosis and ascospore development in nonlinear asci of Neurospora pannonica. Mycologia 94: 99–104 [PubMed] [Google Scholar]
- Rogers JD. (1984) Xylaria acuta, Xylaria cornu-damae and Xylaria mali in continental United States. Mycologia 76: 23–33 [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung GH, Giráldez FL, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny PB, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, Sybren de Hoog G, Crous PW, Hewitt D, Pfister DH, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman AY, Lumbsch HT, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, ÓDonnell K, Sipman H, Rogers JD, Shoemaker RA, Sugiyama J, Summerbell RC, Untereiner W, Johnson PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW. (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239 [DOI] [PubMed] [Google Scholar]
- Seaver FJ. (1930) Photographs and descriptions of cup-fungi X. Ascotremella. Mycologia 22 (2): 51–54 [PubMed] [Google Scholar]
- Seifert KA, Louis-Seize G. (2000) Phylogeny and species concepts in the Penicillium aurantiogriseum complex as inferred from partial beta-tubulin gene DNA sequences. In: Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification (Samson RA, Pitt JI, eds): 189–198 London: Taylor and Francis [Google Scholar]
- Skinner SJ, Tsuneda A, Currah RS. (2006) Morphology and development of the reticuloperidial ascomata of Auxarthron conjugatum. Mycologia 98: 447–454 [DOI] [PubMed] [Google Scholar]
- Spatafora JW, Sung GH, Johnson D, Hesse C, O’Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Miadlikowska J, Reeb V, Gueidan C, Fraker E, Lumbsch T, Lucking R, Schmitt I, Hosaka K, Aptroot A, Roux C, Miller AN, Geiser M, Hafellner J, Hestmark G, Arnold AE, Budel B, Rauhut A, Hewitt D, Untereiner WA, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL. (2006) A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018–1028 [DOI] [PubMed] [Google Scholar]
- Swofford DL. (1998) PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates [Google Scholar]
- Tsui CK, Berbee ML. (2006) Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Molecular Phylogenetics and Evolution 39: 587–597 [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identifcation and mapping of enzymatically amplifed ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Binder M, Schoch CL, Johnston PR, Spatafora JW, Hibbett DS. (2006a) Evolution of helotean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Molecular Phylogenetics and Evolution 41: 295–312 [DOI] [PubMed] [Google Scholar]
- Wang Z, Johnson PR, Takamatsu S, Spatafora JW, Hibbett DS. (2006b) Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98: 1065–1075 [DOI] [PubMed] [Google Scholar]
- Whalley MA, Khalil AMA, Wei TZ, Yao YJ, Whalley AJS. (2010) A new species of Engleromyces from China, a second species in the genus. Mycotaxon 112: 317–23 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds): 315–322 San Diego: Academic Press [Google Scholar]