Abstract
Background:
Various hepatoprotective herbal products from plants are available in Mexico, where up to 85% of patients with liver disease use some form of complementary and alternative medicine. However, only few studies have reported on the biological evaluation of these products.
Objective:
Using a model of carbon tetrachloride (CCl4)-induced hepatotoxicity in rats, we evaluated the effects of commercial herbal extracts used most commonly in the metropolitan area of Monterrey, Mexico.
Materials and Methods:
The commercial products were identified through surveys in public areas. The effect of these products given with or without CCl4 in rats was evaluated by measuring the serum concentrations of aspartate amino transferase (AST) and alanine amino transferase (ALT), and histopathological analysis. Legalon® was used as the standard drug.
Results:
The most commonly used herbal products were Hepatisan® capsules, Boldo capsules, Hepavida® capsules, Boldo infusion, and milk thistle herbal supplement (80% silymarin). None of the products tested was hepatotoxic according to transaminase and histological analyses. AST and ALT activities were significantly lower in the Hepavida+CCl4-treated group as compared with the CCl4-only group. AST and ALT activities in the silymarin, Hepatisan, and Boldo tea groups were similar to those in the CCl4 group. The CCl4 group displayed submassive confluent necrosis and mixed inflammatory infiltration. Both the Hepatisan+CCl4 and Boldo tea+CCl4 groups exhibited ballooning degeneration, inflammatory infiltration, and lytic necrosis. The silymarin+CCl4 group exhibited microvesicular steatosis. The Hepavida+CCl4- and Legalon+CCL4-treated groups had lower percentages of necrotic cells as compared with the CCl4-treated group; this treatment was hepatoprotective against necrosis.
Conclusion:
Only Hepavida had a hepatoprotective effect.
Keywords: Alanine transferase, aspartate transferase, hepatoprotection, liver injury, natural products
INTRODUCTION
Interest in complementary and alternative medicine (CAM) is increasing throughout the world probably because there are only few universally effective and available options for the treatment of common liver diseases such as cirrhosis, fatty liver, and chronic hepatitis.[1,2,3,4,5,6] In recent years, researchers have used scientific methods to evaluate the effects of plants used in traditional CAM for the treatment of liver ailments.[7,8,9,10] In many cases, the mechanisms and modes of action of these plants as well as their therapeutic effectiveness have been confirmed in clinical studies. Several hundred plants have been examined so far, but only a handful have been studied thoroughly. Among these are Silybum marianum (milk thistle), Picrorhiza kurroa (kutki), Curcuma longa (turmeric), Camellia sinensis (green tea), and Glycyrrhiza glabra (licorice).[11,12]
The increasing use of herbal medicines reflects their perceived effectiveness in the treatment and prevention of disease, and the belief that these treatments are safe because they are “natural.” In addition, most countries do not impose strict regulations on herbal preparations, and, therefore, access to these kinds of therapies is almost unrestricted.[13,14]
The use of CAM in the treatment of liver disease varies among different geographic regions. It is estimated that 20–65% of the population in Europe, 39% in the United States, and 85% in Mexico have used CAM for this purpose.[15,16] Several recent surveys from Europe and the United States have demonstrated sharp increases in the use of botanical drugs.[15,17] For example, 21% of patients attending an outpatient liver clinic took herbal preparations and 13% used herbs to treat liver diseases as per a previous study.[15]
The risks of toxicity associated with several commercially available herbal preparations have surfaced recently, challenging the notion that these therapies are innocuous.[18,19] This association between herbal remedies and hepatotoxicity is not a novel finding. It is well known that some plants, particularly the species containing pyrrolizidine derivatives, may be hepatotoxic.[13,20] The risks are particularly high when the therapeutic preparation contains a combination of numerous plants.
The present study was undertaken to evaluate the effects of commercial herbal extracts in the normal liver and in a rat model of acute carbon tetrachloride (CCl4)-induced hepatotoxicity. CCl4 is a chemical used widely to induce liver damage in experimental studies, and its toxicity has been studied extensively. CCl4-induced hepatic injury is characterized by leakage of cellular enzymes into the bloodstream and by centrilobular necrosis.[21,22]
MATERIALS AND METHODS
Identification of commercial herb products
To identify the five most commonly used commercial herbal products employed in the treatment of liver diseases in the region, we administered surveys in various public areas around the metropolitan area of Monterrey, Nuevo León, Mexico. The survey asked the respondents to list all the commercial herbal products of which they were aware.
Chemicals
Sodium chloride solution (0.9%) was obtained from PISA (Guadalajara, Jal, Mexico), and olive oil was obtained from Ybarra SA (Seville, Spain). CCl4 (99.9%) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Legalon® 70 mg silymarin) was obtained from Nycomed (Naucalpan de Juárez, Edo. México, Mexico). Hepatisan capsules (400 mg) were obtained from Herbital (Puebla, Pue, Mexico). Boldo capsules (500 mg) and Hepavida® capsules (400 mg) were obtained from Botnatura (Irapuato, Gto, Mexico). Boldo infusion (1 g) was obtained from Therbal (Mexico City, Mexico). Milk thistle herbal supplement (80% silymarin) was obtained from GNC Herbal Plus (Pittsburg, PA, USA). Pentobarbital sodium was purchased from Holland S.A. de C.V. (Jiutepec, Mor, Mexico).
Animal procedures
Animal procedures were performed in accordance with the Guiding Principles for the Production, Care, and Use of Laboratory Animals of the Mexican Official Norm NOM-062-Z00-1999 published in the Federal Official Diary on August 22, 2001. All protocols used in this study were approved by the ethics committee of our institution. Experiments were performed on male Wistar rats weighing 200–300 g. The animals were maintained under standard conditions, such as stable room temperature (24 ± 3°C), a 12 h light/dark cycle, and access to commercial rat pellets and water ad libitum.
Assessment of toxicity in vivo
The rats were divided into eight groups (n = 5). One group received physiological saline (1 ml/kg, p.o.) and served as the normal control (NC). One group received a CCl4/oil preparation (1:1, 1 ml/kg, i.p.) and served as the acute toxicity control; this group was sacrificed after 24 h to obtain liver and blood samples. One group received Legalon (70 mg/kg, p.o.), a silymarin preparation that was used as the hepatoprotective standard drug. The other groups received one of the following: Hepatisan (800 mg/kg, p.o.), Boldo capsules (1500 mg/kg, p.o.), Hepavida (1200 mg/kg, p.o.), Boldo tea (0.1 ml/kg, p.o.), or silymarin (200 mg/kg, p.o.). The dose of each product was established based on the dose stated for human consumption in the product insert.
To evaluate the potential hepatotoxicity, the six commercial products mentioned above were administered daily for 1 week.[23] This duration of treatment was selected to confirm that the dose to be tested for hepatoprotection was not toxic. At the end of the week, blood and liver samples were collected, and the serum activities of aspartate amino transferase (AST) and alanine amino transferase (ALT) were measured, and liver samples were analyzed histopathologically. Rats were anesthetized with pentobarbital sodium (35 mg/kg, i.p.), and 3 ml of blood was obtained from the abdominal aorta of each rat. Serum was obtained after centrifugation at 3500 rpm for 10 min and was stored at −20°C until enzyme analysis. The livers were dissected carefully and transferred immediately to a 10% formalin solution for fixation. Histopathological analysis was performed on the fixed tissue.[24,25]
Assessment of hepatoprotection in vivo
To evaluate the possible hepatoprotective effect, an additional six groups (n = 5 each) were included: Legalon+CCl4, Hepatisan+CCl4, Boldo capsules+CCl4, Hepavida+CCl4, Boldo tea+CCl4, and silymarin+CCl4. To study the effect of the commercial products on CCl4-induced toxicity, each product was administered at the dose described above for 3 consecutive days, and then CCl4/oil (1:1, 1 ml/kg, i.p.) was administered.[26,27] The rats were sacrificed 24 h after injection of CCl4, and the samples were obtained as described above.
Measurement of serum transaminases
The activities of AST and ALT were measured using an automatic biochemical detection system (DTII Vitro Systems Chemistry; module DTSCII, Johnson and Johnson Ortho-Clinical Diagnostics, New Brunswick, NJ, USA). In the AST assay (21CFR 862.1100), the amino group of l-aspartate is transferred to a-ketoglutarate in the presence of pyridoxal-5-phosphate to produce glutamate and oxaloacetate. The oxaloacetate formed in the deamination of the l-aspartate is converted to malate by malate dehydrogenase in the presence of reduced nicotinamide adenine dinucleotide (NADH), which is oxidized to NAD+. The rate of oxidation of NADH is monitored by reflectance spectrophotometry at 37°C. The rate of change is then used to calculate enzyme activity.[28] In the ALT assay (21 CFR 862.1030), the amino group of l-alanine is transferred to a-ketoglutarate in the presence of pyridoxal-5-phosphate to produce glutamate and pyruvate. The pyruvate formed in the deamination of l-alanine is converted to lactate by lactate dehydrogenase in the presence of NADH, which is oxidized to NAD+. The rate of oxidation of NADH is monitored by reflectance spectrophotometry at 37°C. The rate of change is then used to calculate enzyme activity.[29]
Histology
Liver tissues were embedded in paraffin blocks using conventional methods. The blocks were sectioned at 5 μm and stained with hematoxylin–eosin. The slides were observed under light microscopy to identify histopathological changes including ballooning degeneration, lytic necrosis, confluent necrosis, submassive necrosis, microvesicular steatosis, macrovesicular steatosis, and lymphocytic or mixed inflammatory infiltration. We also calculated the percentage of necrotic cells from the number of necrotic or apoptotic cells observed at three different high power fields (X400); from zones 1–3, 50 cells were counted in each zone.
Statistical analysis
The data are expressed as mean and standard error. The data were analyzed using the Mann–Whitney–Wilcoxon test, Dunnett's test, and Pearson correlation where appropriate. The SPSS statistical software package (v. 16.0; SPSS Inc., Chicago, IL, USA) was used for all analyses. P < 0.05 was considered significant.
RESULTS
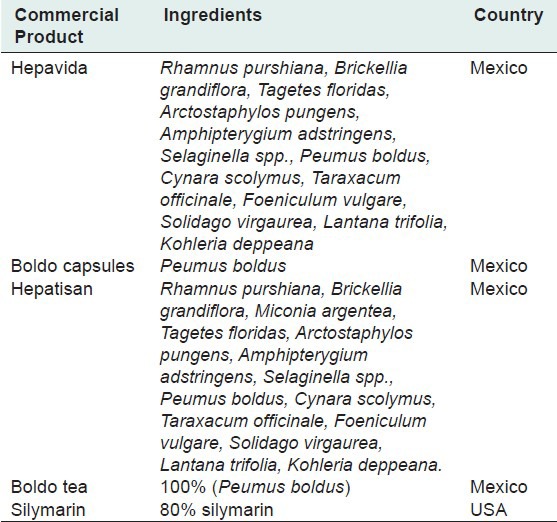
The five most commonly used herbal products for the treatment of liver diseases in the Monterrey metropolitan area are listed in Table 1. Four products were manufactured in Mexico and one in the United States. There was a wide diversity in the constituents of these products. However, four contained Boldo, two of which contained almost exactly the same ingredients [Table 1].
Table 1.
Constituents and sources of commercial products
Assessment of in vivo toxicity
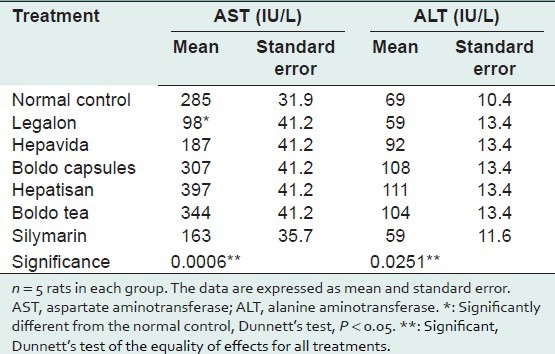
After 7 days of administration, none of the products tested increased ALT and AST activities significantly as compared with the NC group. The ALT and AST activities were significantly lower in the Legalon group than in the NC group (P = 0.0251 and P = 0.0006, respectively) [Table 2]. This suggests that none of the products tested was hepatotoxic, at least when given subacutely.
Table 2.
AST (IU/L) and ALT (IU/L) activities in commercial herbal products compared with the normal control
The livers of the CCl4 group showed severe inflammatory infiltration and necrosis. No other group showed abnormalities in the liver architecture, necrosis, or steatosis. These results indicate that none of the products studied produced hepatotoxicity. Thus, they were all considered for further evaluation of their possible hepatoprotective effects.
Assessment of hepatoprotection in vivo
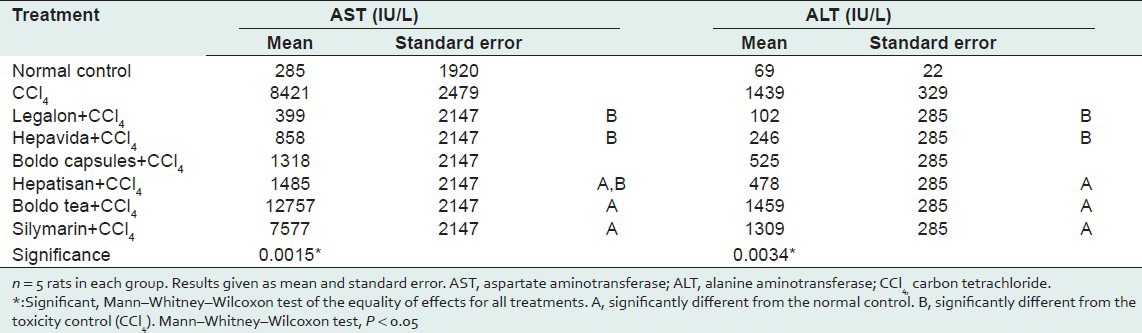
AST and ALT activities were significantly lower in the Hepavida+CCl4 and Legalon+CCl4 groups as compared with the CCl4-only group, and these levels were comparable to those of the NC. The enzyme activities were significantly higher in the groups treated with silymarin, Hepatisan, and Boldo tea than in the NC group; the enzyme activities in the silymarin, Hepatisan, and Boldo tea groups did not differ significantly from those in the CCl4 group [Table 3].
Table 3.
Comparison of AST (IU/L) and ALT (IU/L) activities between treatments with different commercial herbal products as compared with the normal control and CCl4
All animals exhibited hepatocyte ballooning degeneration independent of the treatment received. The CCl4 group showed submassive and confluent necrosis with mixed inflammatory infiltration. The Legalon+CCl4 group exhibited microvesicular steatosis without necrosis, particularly in the perivenular region. Both the Hepatisan+CCl4 and Boldo tea+CCl4 groups exhibited ballooning degeneration, lymphocytic or mixed inflammatory infiltration, and lytic necrosis [Figure 1]. The silymarin group exhibited microvesicular steatosis irrespective of CCl4 administration.
Figure 1.
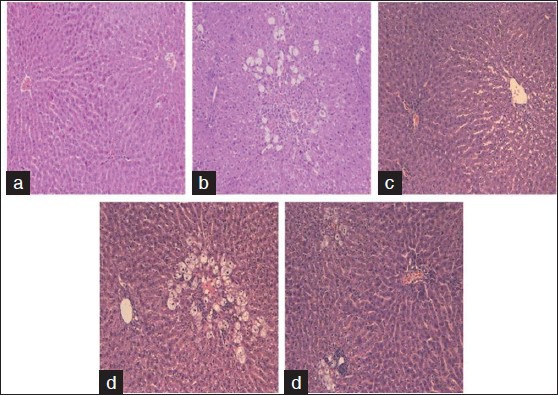
Hematoxylin and eosin staining, X40. (a) Normal control, normal histological findings in the liver parenchyma. (b) CCl4, submassive confluent necrosis and mixed inflammatory infiltration. (c) Legalon+CCl4, perivenular microvesicular steatosis without necrosis. (d) Hepatisan+CCl4, ballooning inflammatory infiltration and lytic necrosis. (e) Boldo tea+CCl4, ballooning degeneration, inflammatory infiltration, and lytic necrosis
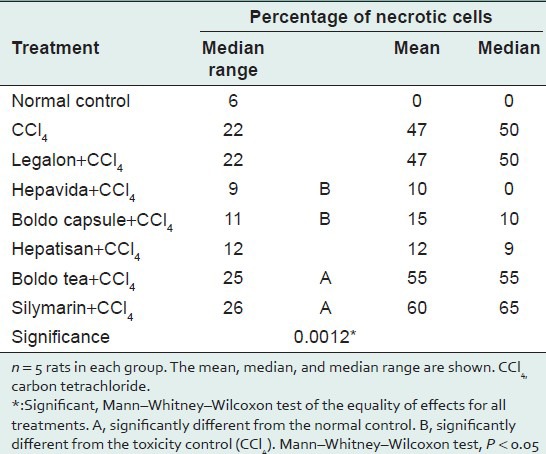
The total percentages of necrotic cells differed significantly between the treatments. The percentages were significantly higher in the Hepatisan, Boldo tea, and silymarin groups than in the NC. The percentages of necrotic cells were significantly lower in the Legalon and Hepavida groups than in the CCl4 group, indicating a hepatoprotective effect against necrosis [Table 4].
Table 4.
Comparison of the percentages of necrotic cells between different commercial herbal products and controls
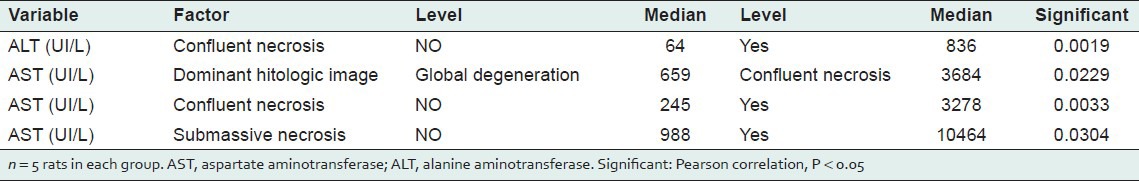
For treatments given without CCl4, the histological changes correlated significantly only with AST and ALT activities (Pearson correlation = 0.739, P < 0.0001). For treatments given with CCl4, ALT and AST activities were positively associated with the presence of confluent necrosis (P = 0.0019 and P = 0.0033, respectively) [Table 5]. AST activity was also positively associated with submassive necrosis (P = 0.030) [Table 5].
Table 5.
Associations between biochemical and histological variables
DISCUSSION
Principal findings
We tested the hepatoprotective effects of five commercial herbal products using a rat model of CCl4-induced hepatotoxicity, comparing them to the reference drug Legalon. We found that none showed hepatotoxic effects in our experimental model and that only Boldo tea caused slight histological inflammation after 7 days of administration. However, only one of these products (Hepavida) showed consistent hepatoprotective effects against CCl4-induced damage. The reference drug Legalon was also hepatoprotective.
Boldo, which comes from a tree in the Monimiaceae family, is believed to be a regulator of hepatic function and an antispasmodic, digestive stimulant, and sedative.[30,31] Four of the products tested contained Boldo, and, of these, only one had a beneficial effect, suggesting that Boldo has poor hepatoprotective properties. Silymarin, a mixture of flavonolignans extracted from seeds of S. marianum, is composed primarily of silibinin, silidianin, and silychristin.[9,32] A survey in patients in a hepatology clinic found that 31% were using over-the-counter “alternative agents” for their liver diseases, the most common being milk thistle (silymarin).[33] In our study, the silymarin extract did not show toxicity at the doses administered, but it failed to show a hepatoprotective effect against CCl4-induced damage in rats. Legalon, a standardized extract of silymarin, which we used as a positive control for hepatoprotection,[34,35,36] showed no toxicity and attenuated the increase in liver enzyme activities in our CCl4 toxicity model. Although the silymarin concentrations in both preparations differed, this suggests that the extract should be analyzed to determine whether it contains the amounts of silymarin stated on the label and how these differ from the amount contained in Legalon.
Strenghts and weaknesses of the study
We used a well-studied model of hepatotoxicity (CCl4) and used both biochemical and histological markers of liver injury. In addition, we compared the effects of the commercial products against the reference drug Legalon, a drug with established hepatoprotective properties.
There are some weaknesses in our study. Our sample size was small (5 animals per group), a fact that could account for the large standard errors we obtained in our data. We also did not objectively test the commercial products in order to confirm that they possessed the contents that were reported in the label by the manufacturer. There are also inherent complications in identifying which of the many components in a commercial mixture of herbs are responsible for its biological effects, if any, are found.
Relation to other studies
We know of no other studies that have analyzed the hepatoprotective effects of these commercial products. As mentioned above, Legalon is of established efficacy. Several studies have reported that the major histopathological parameters altered with CCl4-induced damage are degeneration in hepatocytes, the presence of necrosis and inflammatory infiltration, and elevated ALT and AST activities.[37,38] Our histology analyses showed a positive association between elevated ALT and AST activities and the presence of confluent necrosis and submassive necrosis in the groups that received commercial products, which were ineffective in attenuating the increase in enzyme activities caused by CCl4 treatment.
Implications
A wide variety of herbal products are available in Mexico, many without the official approval of the Secretary of Health. It has been reported that 60–80% of the general population uses these products, and up to 85% of patients with liver disease use some form of CAM.[39] There are reported cases of acute liver disease, at times severe, associated with the consumption of herbal products, and some products have been withdrawn from the market based on recommendations made by national pharmacovigilance agencies.[13,40] However, consumption of these products is increasing.
In this paper, we studied five herbal products used commonly in the region.
Consistency in biological activity and composition are essential to ensuring the safety of therapeutic agents. However, botanical preparations rarely meet this standard because of problems identifying plants, variable growing conditions, differences in the processing of extracts, and lack of information about the pharmacologically active ingredients. The use of marker compounds to standardize herbal preparations promotes batch-to-batch consistency but does not ensure consistent pharmacological activity. Moreover, analyses of purportedly standardized herbal preparations show that botanical products often do not contain the amounts of the compounds stated on the label.[41] It is possible that some of these factors contributed to the results obtained in this study. For example, both Hepavida and Hepatisan supposedly contain the same compounds, yet only Hepavida was hepatoprotective. A similar finding was obtained with products that contain Boldo; only one was hepatoprotective in our model. This suggests that the presence of the active ingredient (boldine) in this plant should be confirmed in the commercial products, given the variability of the effects of the different preparations.
As suggested above, there is a need for quality control in laboratories that produce botanical products used in the treatment of diseases, and the presence of active pharmacological components should be confirmed. Reporting and regulatory systems should be created to monitor adverse effects of herbal products, and care should be taken in the identification of compounds and in the evaluation of the potential benefits of phytotherapy. In conclusion, only Hepavida had a hepatoprotective effect in our model.
For future research
Simple methods could be used to test commercial products for their advertised hepatoprotective effects, given that they are usually poorly regulated. However, our results only apply to relatively short periods of administration. Further research is needed to establish if the products tested here might have hepatoprotective effects after chronic use (months to years) or if they have liver regenerative properties. Evidently, their clinical value could only be established with human studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 2.Kashi MR, Torres DM, Harrison SA. Current and emerging therapies in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:396–406. doi: 10.1055/s-0028-1091984. [DOI] [PubMed] [Google Scholar]
- 3.Lam BP, Younossi ZM. Treatment regimens for non-alcoholic fatty liver disease. Ann Hepatol. 2009;8:S51–9. [PubMed] [Google Scholar]
- 4.Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis. 2009;29:211–21. doi: 10.1055/s-0029-1214376. [DOI] [PubMed] [Google Scholar]
- 5.Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174–84. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevaliez S, Pawlotsky JM. How to use virological tools for optimal management of chronic hepatitis C. Liver Int. 2009;29:S9–14. doi: 10.1111/j.1478-3231.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 7.Azzam HS, Goertz C, Fritts M, Jonas WB. Natural products and chronic hepatitis C virus. Liver Int. 2007;27:17–25. doi: 10.1111/j.1478-3231.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- 8.Luk JM, Wang X, Liu P, Wong KF, Chan KL, Tong Y, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: From laboratory discovery to clinical evaluation. Liver Int. 2007;27:879–90. doi: 10.1111/j.1478-3231.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 9.Ball KR, Kowdley KV. A review of Silybum marianum (milk thistle) as a treatment for alcoholic liver disease. J Clin Gastroenterol. 2005;39:520–8. doi: 10.1097/01.mcg.0000165668.79530.a0. [DOI] [PubMed] [Google Scholar]
- 10.Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: A systematic review. J Viral Hepat. 2005;12:559–67. doi: 10.1111/j.1365-2893.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 11.Luper S. A review of plants used in the treatment of liver disease: Part 1. Altern Med Rev. 1998;3:410–21. [PubMed] [Google Scholar]
- 12.Luper S. A review of plants used in the treatment of liver disease: Part 2. Altern Med Rev. 1999;4:178–88. [PubMed] [Google Scholar]
- 13.Larrey D. Hepatotoxicity of herbal remedies. J Hepatol. 1997;26:47–51. doi: 10.1016/s0168-8278(97)82333-1. [DOI] [PubMed] [Google Scholar]
- 14.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 15.Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, et al. Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol. 2002;97:2391–7. doi: 10.1111/j.1572-0241.2002.05993.x. [DOI] [PubMed] [Google Scholar]
- 16.Taddei-Bringas GA, Santillana-Macedo MA, Romero-Cancio JA, Romero-Tellez MB. Acceptance and use of medicinal plants in family medicine [Article in Spanish] Salud Publica Mex. 1999;41:216–20. [PubMed] [Google Scholar]
- 17.Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262–8. doi: 10.7326/0003-4819-135-4-200108210-00011. [DOI] [PubMed] [Google Scholar]
- 18.Larrey D, Vial T, Pauwels A, Castot A, Biour M, David M, et al. Hepatitis after germander (Teucrium chamaedrys) administration: Another instance of herbal medicine hepatotoxicity. Ann Intern Med. 1992;117:129–32. doi: 10.7326/0003-4819-117-2-129. [DOI] [PubMed] [Google Scholar]
- 19.MacGregor FB, Abernethy VE, Dahabra S, Cobden I, Hayes PC. Hepatotoxicity of herbal remedies. BMJ. 1989;299:1156–7. doi: 10.1136/bmj.299.6708.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valla D, Benhamou JP. Drug-induced vascular and sinusoidal lesions of the liver. Baillieres Clin Gastroenterol. 1988;2:481–500. doi: 10.1016/0950-3528(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 21.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 22.Janakat S, Al-Merie H. Optimization of the dose and route of injection, and characterisation of the time course of carbon tetrachloride-induced hepatotoxicity in the rat. J Pharmacol Toxicol Methods. 2002;48:41–4. doi: 10.1016/S1056-8719(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 23.Morita M, Akai S, Hosomi H, Tsuneyama K, Nakajima M, Yokoi T. Drug-induced hepatotoxicity test using gamma-glutamylcysteine synthetase knockdown rat. Toxicol Lett. 2009;189:159–65. doi: 10.1016/j.toxlet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Cámara-Lemarroy CR, Guzmán-de la Garza FJ, Cordero-Pérez P, Alarcón-Galván G, Torres-Gonzalez L, Muñoz-Espinosa LE, et al. Comparative effects of triflusal, S-adenosylmethionine, and dextromethorphan over intestinal ischemia/reperfusion injury. Scientific World Journal. 2011;11:1886–92. doi: 10.1100/2011/583603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzmán-de la Garza FJ, Cámara-Lemarroy CR, Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa LE, Fernández-Garza NE. Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol. 2009;15:3901–7. doi: 10.3748/wjg.15.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine. 2007;14:129–35. doi: 10.1016/j.phymed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari P, Kumar A, Ali M, Mishra KP. Radioprotection of plasmid and cellular DNA and Swiss mice by silibinin. Mutat Res. 2010;695:55–60. doi: 10.1016/j.mrgentox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Reference Method for AST. Manual Reference Method: 21 CFR Part 862. Section 1110 [Google Scholar]
- 29.Reference Method for ALT. Manual Reference Method: 21 CFR Part 862. Section 1030 [Google Scholar]
- 30.Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY, Shyu JC, et al. Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J Viral Hepat. 2008;15:508–14. doi: 10.1111/j.1365-2893.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 31.Orhan DD, Aslan M, Aktay G, Ergun E, Yesilada E, Ergun F. Evaluation of hepatoprotective effect of Gentiana olivieri herbs on subacute administration and isolation of active principle. Life Sci. 2003;72:2273–83. doi: 10.1016/s0024-3205(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Vol. 1. Geneva: WHO; 2002. WHO Traditional Medicine Strategy 2002–2005; pp. 1–61. [Google Scholar]
- 33.Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. doi: 10.1055/s-2002-30104. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz O, Montes M, Wilkomirsky T. Santiago: Editorial Universitaria; 2001. Plantas Medicinales de uso en Chile; Química Y Farmacología; pp. 223–6. [Google Scholar]
- 35.Fernandez J, Lagos P, Rivera P, Zamorano-Ponce E. Effect of boldo (Peumus boldus Molina) infusion on lipoperoxidation induced by cisplatin in mice liver. Phytother Res. 2009;23:1024–7. doi: 10.1002/ptr.2746. [DOI] [PubMed] [Google Scholar]
- 36.Lanhers MC, Joyeux M, Soulimani R, Fleurentin J, Sayag M, Mortier F, et al. Hepatoprotective and anti-inflammatory effects of a traditional medicinal plant of Chile, Peumus boldus. Planta Med. 1991;57:110–5. doi: 10.1055/s-2006-960043. [DOI] [PubMed] [Google Scholar]
- 37.Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus) Fitoterapia. 1995;66:3–42. [Google Scholar]
- 38.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–43. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 39.Orhan DD, Orhan N, Ergun E, Ergun F. Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. J Ethnopharmacol. 2007;112:145–51. doi: 10.1016/j.jep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal M, Golberg A, Brinckmann J. The complete German Commission E monographs. Therapeutic Guide to Herbal Medicine. 2000;1:38–40. [Google Scholar]
- 41.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen Pharmacol. 1999;32:661–7. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]


