Abstract
Background:
Terminalia chebula is called the “king of medicines” in Tibet and is always listed first in the Ayurvedic meteria medica because of its extraordinary powers of healing.
Objective:
Identification, isolation and screening of pyrogallol which are responsible for antimicrobial property of fruits of Terminalia chebula.
Materials and Methods:
Ethyl acetate fraction of fruits of Terminalia chebula was subjected to Gas chromatography–mass spectrometry (GC-MS) for the components present in the extract.
Results:
Sixty four constituents were identified out of which kaempferol-3-O-rutinoside flavonoid and Vitamin E has been detected for the first time in fruits of this plant. Pyrogallol (46.26%) which was the major component of the extract in GC-MS analysis was isolated and screened for antimicrobial activity against selected test pathogens by Disc Diffusion Assay. Crude ethyl acetate fraction of the fruits was showing the same activity potential as was observed for pure pyrogallol which was the major component as per GC-MS analysis. The most sensitive species among the bacteria was Enterobacter aerogenes with highest inhibition zone (IZ = 31 mm; AI = 1.409 ± 0.046) even at minimum inhibitory concentration (0.039 mg/ml).
Conclusion:
Hence activity shown by crude ethyl acetate fraction might be due to pyrogallol present in the extract. On the basis of results it can be advocate that achieved crude ethyl acetate fraction can be explored for preparing antimicrobial drugs in future for the infectious caused by the pathogens tested in the study.
Keywords: Disc diffusion assay, Enterobacter aerogenes, GC-MS, kaempferol-3-O-rutinoside, pyrogallol, Terminalia chebula
INTRODUCTION
Terminalia chebula Retz is a medicinal plant belonging to family Combretaceae. It is commonly called as black myrobalan. The fruits of T. chebula are commonly used in treatment of various ailments such as allergy, vomiting, urinary tract infections, cardiac diseases, digestive problems, bleeding, cancer, skin disorders and diabetes mellitus.[1] It also possesses antioxidant activity and free radical scavenging property. Antimicrobial activitiy of T. chebula have also been reported in many research publications.[2,3] Pyrogallol (benzene-1,2,3-triol) is a polyphenol is known to display fungicidal/fungistatic properties.[4] Moreover, its derivatives are biologically active components of plants and plant products.[5,6] Current study involves the GC-MS analysis of the ethyl acetate extracts of fruits of T. chebula, selection of most active compound identification and screening for antimicrobial activity against selected pathogens. The aim was to determine whether the activity of the plant species is due to individual compound or group of compounds, in addition the aim was to isolate to the most appropriate economical method of extracting the active fraction from fruits of T. chebula which is widely used commercially for herbal medicine.
MATERIALS AND METHODS
Plant material
Fruits of T. chebula were collected in the month of October from the University of Agriculture Sciences Gandhi Krishi Vignyan Kendra, Bangalore and the specimen of the plant was identified at the Department of Botany, University of Rajasthan. The sample specimen with No. RUBL20868 was submitted in the ‘Herbarium’ of Botany Department, University of Rajasthan.
Extraction procedure
Fruits were separately shade dried and finely powered using a mixer. Twenty grams of finely powdered sample was soxhlet extracted with ethyl acetate on a water bath for 24 h and filtered. Obtained extract was dried in vacuum and stored at 4°C. The chemical composition of the ethyl acetate fractions were got analyzed by GC∕MS.
Gas chromatography-mass spectrometry analysis (GC-MS)
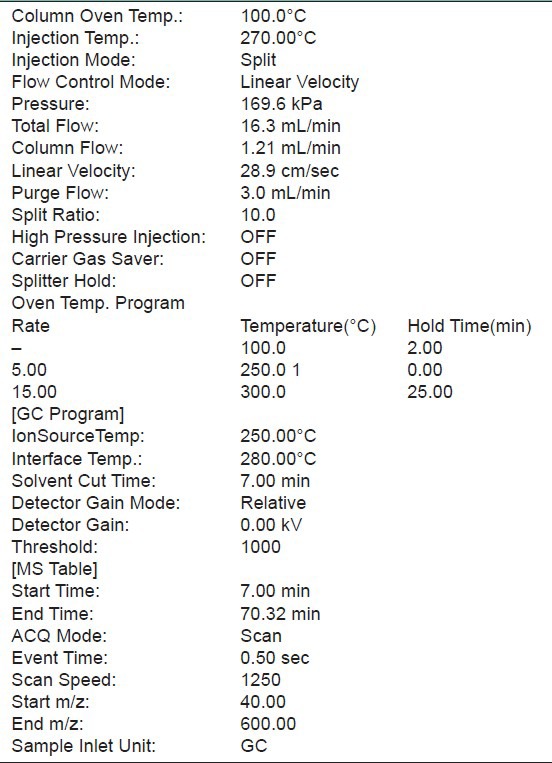
GC-MS technique was used in this study to identify the phytocomponents present in the extracts. This was carried out at Jawaharlal Nehru University, New Delhi, India. The GC-MS used was a Schimadzu QP2010PLUS system. All the conditions used in GC-MS method were recorded in Table 1. Identification of the peaks was based on computer matching of the mass spectra with the National Institute of Standards and Technology (NlST 08 and NIST 08s) library and by direct comparison with published data.[7]
Table 1.
GC-MS method
Identification of pyrogallol (phenol) by chromatography
Dried ethyl acetate extract of fruits dissolved in ethyl acetate, was chromatographed two-dimensionally on silica gel coated (0.2-0.3 mm) plates. These plates were developed in an organic solvent mixture of acetic acid- chloroform (1:9) and ethyl acetate-benzene (9:11) and separately on cellulose MN300 in benzene-methanol-acetic acid (45:8:4) and 6% aqueous acetic acid.[8] One spot (Rf 0.8) in one direction and (Rf 0.15) second direction was observed which indicate the presence of pyrogallol in the ethyl acetate extracts of fruit. Preparative TLC of the extract was carried out on silica gel coated and activated (0.4-0.5-mm thick) glass plates in the selected solvents. Spot was marked in each plate and was collected and eluted with ethyl acetate. Elutes were pooled, dried in vacuum and rechromatographed to test the purity of the isolated compound.
The isolated compound was crystallized, weighed and subjected to melting point and infra-red spectral studies on Perkins Elmer model 555 spectrophotometer in KBr pellets. Pyrogallol (formula C6H6O3; m.w. 126; m.p. 131° - 135°) was identified in the fruit of plant.
ANTIMICROBIAL ASSAY
Selected test microorganisms
Pathogenic microorganisms selected for study include seven bacteria, viz., Escherichia coli (MTCC no. 46), Pseudomonas aeruginosa (MTCC 1934), Proteus mirabilis (MTCC 3310), Raoultella planticola (MTCC 2271), Enterobacter aerogens (MTCC 2822), Bacillus subtilis (MTCC 121), Staphylococcus aureus (MTCC 3160) and three fungal strains, viz., Candida. albicans (MTCC 183), Aspergillus flavus (MTCC 277) and Aspergillus niger (MTCC 282). Selected microorganisms were procured from IMTECH, Chandigarh, India. Bacterial strains were grown and maintained on “Muller- Hinton Agar Medium” (Beef extract 2.0 g; Peptone 17.5 g; Starch 1.5 g; Agar 17.0 g; in 1000 ml of distilled water; Final pH 7.4 ± 0.2) at 37 ± 2° while fungal strains were grown on “Sabouraud Dextrose Agar Medium” (Peptone 10 g; Dextrose 20 g; Agar 20 g in 1000 ml of distilled water; pH adjusted to 6.8-7.0 at 27 ± 2°C).
Screening
Disc diffusion assay (DDA) was performed for antimicrobial screening.[9] MH agar (for bacteria) and SD agar (for fungi) base plates were seeded with the standard inoculum size of bacteria, yeast and fungi (1 × 108 CFU/ml for bacteria, 1 × 107 CFU/ml for yeast and 1 × 106 CFU/ml for dermatophytic fungi). Sterile filter paper discs (6 mm in diameter) were impregnated with 100 μl of each of the extract (10 mg/ml concentration) to give a final concentration of 1 mg/disc, left to dry in vaccuo to remove residual solvent, which might interfere with the determination. Extract discs were then placed on the seeded agar plates. Each extract was tested in triplicate along with standard drugs streptomycin (1 mg/disc) for bacteria, itraconazol (1 mg/ml) for A. niger and A. flavus and Clotrimazole (1 mg/ml) for C. albicans, respectively. The plates were kept at 4°C for 1 h for diffusion of extract, thereafter were incubated at 37 ± 2°C for 24 h; 27 ± 2°C for 48 h and 27 ± 2°C for 5-7 days for bacteria, yeast and fungus, respectively. Zone of inhibition (IZ) or depressed growth of microorganisms was measured and the ‘Activity Index’ (AI) for each extract was calculated.
Minimum inhibitory concentration and minimum bactericidal/fungicidal concentration
Minimum inhibitory concentration (MIC) was determined for plant extract showing antimicrobial activity against test pathogens in disc diffusion assay. Broth microdilution method was followed for determination of MIC values.[10] Plant extracts were resuspended in acetone (which has no activity against test microorganisms) to make 10 mg/ml final concentration and then was added to broth media of 96-wells of microtiter plates using two-fold serial dilution. Thereafter, 100-μl inoculum of standard size was added to each well. Bacterial and fungal suspensions were used as negative control, while broth containing standard drug was used as positive control. The microtiter plates were incubated at 37 ± 2°C for 24 h for bacteria, 27 ± 2°C for 48 h for yeast and 27 ± 2°C for 5-7 days for fungi. Each extract was assayed in duplicate and each time two sets of microtiter plates were prepared, one was kept for incubation while another set was kept at 4°C for comparing the turbidity in the wells of microtiter plate. The MIC values were taken as the lowest concentration of the extracts in the well of the microtiter plate that showed no turbidity after incubation. The turbidity of the wells in the microtiter plate was interpreted as visible growth of microorganisms. The minimum bacterial/fungicidal concentration (MBC/MFC) was determined by subculturing 50 μl from each well showing no apparent growth. Least concentration of extract showing no visible growth on subculturing was taken as MBC/MFC.
RESULTS
Phytochemical analysis
The results of GC-MS analysis of the ethyl acetate fraction of fruits of T. chebula identified the various compounds through the NIST08L [database are listed in Table 2]. The active principle, area of the peak concentration (%), retention time (RT), molecular weight and molecular formula are presented in the table. Figure 1 represents the gas chromatograms of the extract which shows 64 distinct peaks identified in GC-MS. The major components in the ethyl acetate fraction as identified by GC-MS was pyrogallol (1,2,3-benzenetriol). Mass spectrum of pyrogallol is shown in Figure 2. The GC-MS spectrum gives the structure of the compound, molecular formula (C6H6O3), molecular weight 126.0 [Figure 2].
Table 2.
Components identified in ethyl acetate fraction of fruits of T. chebula by GC-MS analysis
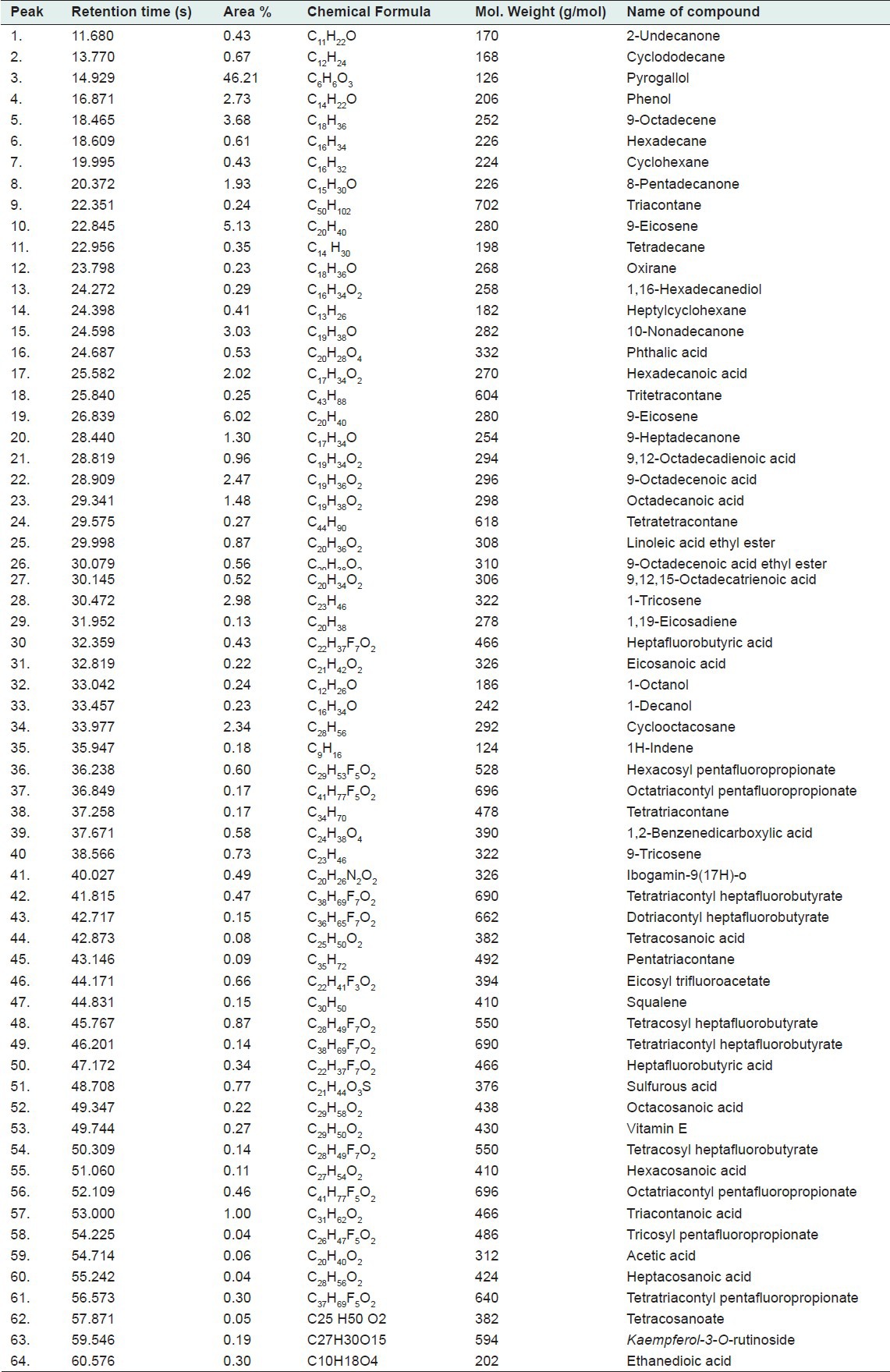
Figure 1.
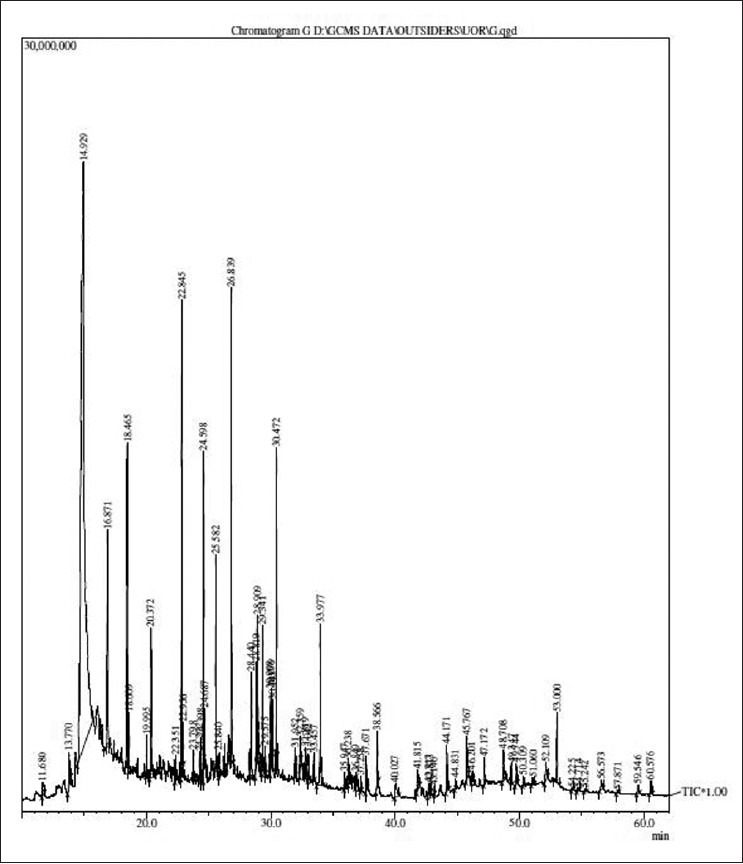
Chromatogram of ethyl acetate fraction of fruits of T. chebula by GC-MS analysis
Figure 2.
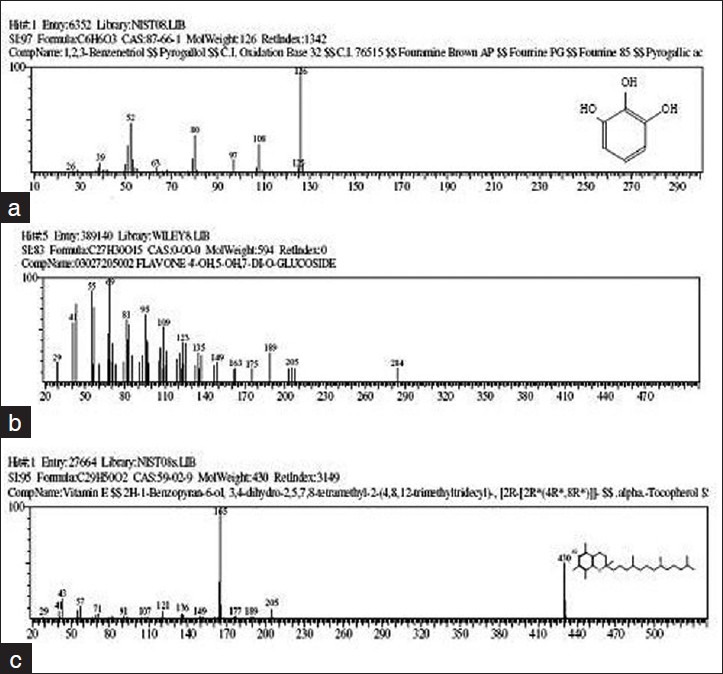
Mass spectrums (a) The GC-MS spectrum gives the structure of the pyrogallol, molecular formula (C6H6O3), molecular weight 126.0 (b) Mass spectrum of Kaempferol-3-O-rutinoside (c) The GC-MS spectrum gives the structure of the vitamin E, molecular formula (C29H50O2), molecular weight 430
A new flavonoid Kaempferol-3-O-rutinoside was identified for the first time in the fruits. Other compounds identified in the extracts are Phenol (2.73%), 9-Octadecene (3.68%), 9-Eicosene (5.13%), Hexadecanoic acid (2.02%), 9,12-Octadecadienoic acid (0.96%), 9-Octadecenoic acid (2.47%), Eicosanoic acid (0.22%), 1,2-Benzenedicarboxylic acid (0.58%), Tetracosanoic acid (0.08%), Vitamin E (0.27%), Ethanedioic acid (0.30%). Figure 3 shows the chemical structure of pyrogallol, kaempferol-3-O-rutinoside and vitamin E.[11] observed gallic acid, chebulic acid, 1,6-di-O-galloyl-D-glucose, punicalagin, 3,4,6-tri-O-galloyl-D-glucose, casuarinin, chebulanin, corilagin, neochebulinic acid, terchebulin, ellagic acid, chebulagic acid, chebulinic acid, and 1,2,3,4,6-penta-O-galloyl-D-glucose) in the fruit of T. chebula Retz. by RP-HPLC method. Tannins contain phenolic carboxylic acid like gallic acid, ellagic acid, chebulic acid and gallotannins such as 1,6 di-Ogalloyl- β-D-glucose, 3,4,6 tri-O-galloyl-β-D-glucose, 2,3,4,6 tetra-O-galloyl-β-D-glucose, 1,2,3,4,6 penta-Ogalloyl- β-D-glucose. Ellagitannin such as punacalagin, casurarinin, corilagin and terchebulin and others such as chebulanin, neochebulinic acid, chebulagic acid and chebulinic acid reported in literature.[12]
Figure 3.
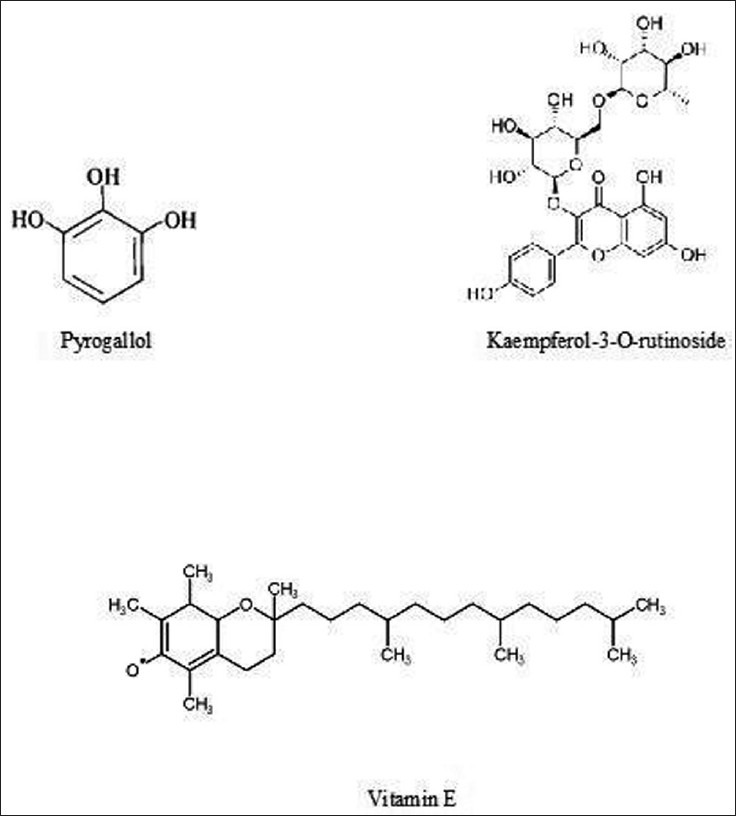
Chemical structure of molecules in ethyl acetate fraction of fruits of T. chebula
Antimicrobial screening
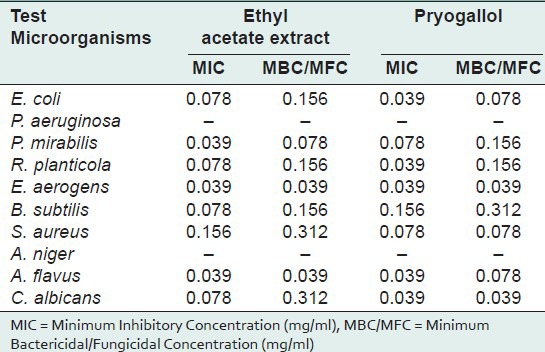
Present investigation clearly indicates the presence of highest percentage of pyrogallol in ethyl acetate fraction of fruits of T. chebula. Pyrogallol has been reported to have various biological activity like allelochemic, antibacterial, abortifacient, anticlastogen, antidermatitic, antilupus, antimutagenic, antioxidant, antipsoriac, antiseptic, CNSActive, candidicide, cardiovascular, ecbolic, fungicide, insulin-sparing, irritant, nephrotoxic, nigrifacient, pesticide, prostaglandin-synthesis-inhibitor from Dr. Duke's phytochemical and ethnobotanical database.[13] Pyrogallol present in the ethyl acetate fraction was identified and eluted by PTLC screened for antimicrobial activity along with ethyl acetate fraction. Antimicrobial activity (assessed in terms of inhibition zone and activity index) of the plant extracts, tested against selected microorganisms was recorded in Table 3. Results reveal that the inhibition zone produced by pyrogallol against selected pathogens was similar to the ethyl acetate fraction of the fruits. In both cases the highest activity was showed against E. aerogens (Pyrogallol: IZ = 31 mm, AI 0. 1.409 ± 0.046; Ethyl acetate fraction: IZ = 29 mm, AI = 1.313 ± 0.026). Against P. mirabilis both pyrogallol and ethyl acetate fraction showed same activity. (IZ = 19 mm, AI = 0.760 ± 0.061). Both the test extracts are not active against P. aeruginosa and A. niger. MIC and MBC/MFC values were evaluated for pyrogallol and ethyl acetate fraction (shown activity in “Disc Diffusion Assay”) and recorded in Table 4.
Table 3.
Antimicrobial activity of crude ethyl acetate extract and pure pyrogallol of T. chebula by Disc Diffusion Assay
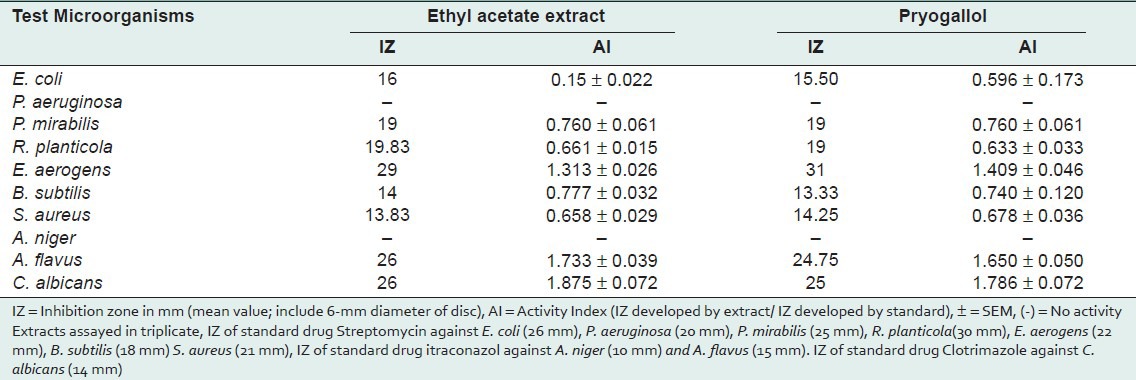
Table 4.
MIC and MBC/MFC values of crude ethyl acetate extract and pure pyrogallol of T. chebula by Disc Diffusion Assay
Discussions and conclusions
In the present investigation MIC 0.039 mg/ml was recorded against P. mirabilis, E. aerogens, A. flavus by ethyl acetate fraction and against E. coli, R. planticola, E. aerogens, A. flavus, C. albicans by pyrogallol. Pyrogallol (1,2,3- Trihydroxybenzene) is allochemical which contains 3 hydroxyl groups belong to phenolic compounds of plants. The phenolic hydroxyl group has a wide range of cellular activities that have not been clearly investigated. At present there is intense interest in polyphenols which are present in the diet as part of fruits, tea, coffee and wine[14] since they have been shown to protect cells from oxidative stress.[15] In addition, these compounds show a wide spectrum of action involving antitumor, antivirial, antibacterial, cardioprotective, prooxidant and antimutagenic activity.[16,17] The presence of the hydroxyl group and a system of delocalized electron play an important role in the antimicrobial activity.[18] A characteristic feature of a phenolic hydroxyl group is its significantly greater acidity than that of an aliphatic hydroxyl groups.[19] Hydroxyl group and a system of delocalized electrons might be responsible for strong antimicrobial activity. It was described earlier that the hydroxyl group (bound to a benzene ring) is important for the activities of some antimicrobial compounds and that these activities are enhanced by the presence of alpha-beta double bonds.[20] Pyrogallol has been reported to be an effective antimicrobial agent and its toxicity is attributed to the three hydroxyl groups present in its structure.[21,22]
In the conclusion, it is showed that the antimicrobial activity of ethyl acetate fraction was due to pyrogallol which is present in higher quantity in fruits. Results of the present study reveal that all compound tested, inhibited the growth of selected bacteria and fungi, indicating broad spectrum bioactive nature of selected plant. In the present scenario when existing antibiotics are gradually becoming ineffective against pathogenic microorganisms, such studies should highly be encouraged, so that new and alternative sources for future antibiotics may be explored well in advance.
ACKNOWLEDGMENTS
Authors are thankful to the Head of Botany Department, University of Rajasthan for providing all necessary facilities for present work. Financial assistance provided by UGC is gratefully acknowledged.
Footnotes
Source of Support: UGC
Conflict of Interest: Department of Botany, University of Rajasthan
REFERENCES
- 1.Chatttopadhyay RR, Bhattacharyya SK. Terminalia chebula: An update. Pharmacogn Rev. 2007;1:151–6. [Google Scholar]
- 2.Kim HG, Cho JH, Jeong EY, Lim JH, Lee SH, Lee HS, et al. Growth inhibitory activity of active component from Terminalia chebula fruits against intestinal bacteria. J Food Prot. 2006;69:2205–9. doi: 10.4315/0362-028x-69.9.2205. [DOI] [PubMed] [Google Scholar]
- 3.Vonshak A, Barazani O, Sathiyomoorthy P, Shalev R, Vardy D, Golan-Goldhirsh A. Screening South Indian medicinal plants for antifungal activity against cutaneous pathogens. Phytother Res. 2003;17:1123–5. doi: 10.1002/ptr.1399. [DOI] [PubMed] [Google Scholar]
- 4.Shukla YN, Srivastava A, Kumar S, Kumar S. Phytotoxic and antimicrobial constituents of Argyreia speciosa and oenothera biennis. J Ethnopharmacol. 1999;67:241–5. doi: 10.1016/s0378-8741(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 5.Koga T, Moro K, Nakamori K, Yamakoshi J, Hosoyama H, Kataoka S, et al. Increase of antioxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seeds. J Agric Food Chem. 1999;47:1892–7. doi: 10.1021/jf9810517. [DOI] [PubMed] [Google Scholar]
- 6.Burns J, Gardner PT, O’Neil J, Crawford S, Morecroft I, McPhail DB, et al. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem. 2000;48:220–30. doi: 10.1021/jf9909757. [DOI] [PubMed] [Google Scholar]
- 7.Vandendool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–71. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 8.Harborne JC. 2nd ed. London, New York: Chapman and Hall Ltd; 1984. Phytochemical methods: A guide to modern techniques of plant analysis. [Google Scholar]
- 9.Andrews JM. BSAC standardized disc susceptibility testing method. J Antimicrob Chemother. 2001;4:43–57. doi: 10.1093/jac/48.suppl_1.43. [DOI] [PubMed] [Google Scholar]
- 10.Barsi DF, Fan SH. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J Pharmacol. 2005;37:26–9. [Google Scholar]
- 11.Juang LJ, Sheu SJ, Lin TC. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J Sep Sci. 2004;27:718–24. doi: 10.1002/jssc.200401741. [DOI] [PubMed] [Google Scholar]
- 12.Lin-Jeng J, Shuenn-Jyi S, Ta-Chen L. Determination of hydrolysable tannins in the fruit of Terminalia chebula Retz. by highperformance liquid chromatography and capillary electrophoresis. J Sep Sci. 2004;27:718–24. doi: 10.1002/jssc.200401741. [DOI] [PubMed] [Google Scholar]
- 13.Sangeetha J, Vijayalakshmi K. Determination of bioactive components of ethyl acetate fraction of Punica granatum rind extract. Int J Pharm Sci Drug Res. 2011;3:116–22. [Google Scholar]
- 14.Moridani MY, Scobie H, O’Brien PJ. Catechin metabolism: Glutathione conjugate formation catalysed by tyrosinase, peroxidase, and cytochrome 450. Chem Res Toxicol. 2001;14:841–8. doi: 10.1021/tx000235o. [DOI] [PubMed] [Google Scholar]
- 15.Skaper SD, Fabris M, Ferrari V, Daile Carbonare M, Leon A. Quercetin protects cutaneous tissue-associated cell type including sensory neurons from oxidative stress induced by glutathione depletion: Cooperative effects of ascorbic acid. Free Radic Biol Med. 1997;22:669–78. doi: 10.1016/s0891-5849(96)00383-8. [DOI] [PubMed] [Google Scholar]
- 16.Rice-Evans CA, Miller NJ, Paganga G. Structureantioxidant activity relationship of flavonoids and phenolic acid. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate show a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;1129:173–9. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 18.Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002;68:1561–8. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown WH. 3rd ed. Boston, Mass: Willard Grant Press; 1975. Introdiction to orgainic chemistry. [Google Scholar]
- 20.Shelef LA. Antimicrobial effects of spices. J Food Saf. 1983;6:29–44. [Google Scholar]
- 21.Kocacaliskan I, Talan I, Terzi I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z Naturforsch C. 2006;61:639–42. doi: 10.1515/znc-2006-9-1004. [DOI] [PubMed] [Google Scholar]
- 22.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]


