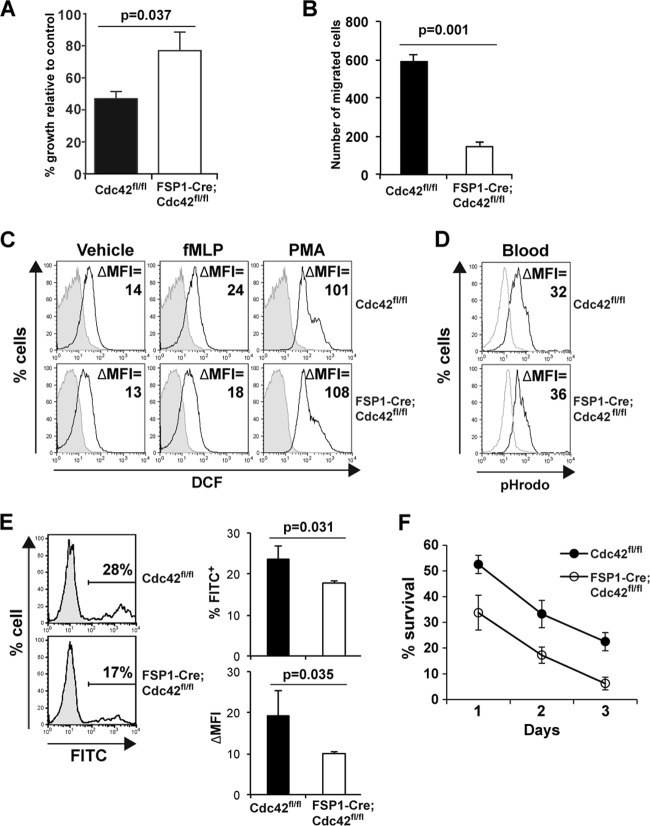
Fig 6.
Cdc42 is required for normal killing activity, migration, and survival of neutrophils. (A) Reduced antimicrobial activity of Cdc42-deficient neutrophils. Purified neutrophils were incubated with S. aureus precoated with complement, after which bacterial viability was determined by plating of serial dilutions on solid medium. Shown are the percentages of bacterial growth after incubation with neutrophils relative to the number of bacteria incubated in the absence of neutrophils. Data represent the mean ± SEM of six Cdc42fl/fl mice and six FSP1-Cre;Cdc42fl/fl mice. (B) Impaired migration of Cdc42-deficient neutrophils. Purified neutrophils were plated in the upper chamber with a 5-μm-pore-size polycarbonate membrane coated with fibronectin and stained with crystal violet after 4 h of incubation. Cells in 10 independent high-powered fields were counted and averaged. (C) Cdc42 is dispensable for ROS production of neutrophils. Bone marrow cells were stimulated with N-formyl-methionyl-leucyl-phenylalanine (fMLP), phorbol myristate acetate (PMA), or vehicle for 30 min and incubated with H2DCFDA and antibodies against specific surface markers. Shown are representative histograms of Gr1+ CD11b+ gated populations. Three independent experiments were performed. The changes in the mean fluorescence intensity (ΔMFI) were calculated by subtracting the mean fluorescence intensity of unstained controls (shaded) from the mean fluorescence intensity of DCF (line). (D) Cdc42 is dispensable for phagosomal acidification of neutrophils. Acidification of blood neutrophils was measured using a pHrodo E. coli Bioparticles phagocytosis kit. Shown are representative histograms of two independent experiments. (E) Impaired phagocytic endocytosis of Cdc42-deficient neutrophils. Purified neutrophils were incubated with FITC-conjugated latex beads, and endocytosis was measured by flow cytometry. Shown are representative histograms of viable granulocyte populations (shaded histogram, neutrophil alone; line histogram, neutrophils incubated with the beads). The mean frequency of phagocytic cells (percentage of FITC-positive cells) and phagocytic activity (ΔMFI = mean fluorescence intensity of experimental sample − mean fluorescence intensity of neutrophils alone) for five Cdc42fl/fl mice and five FSP1-Cre;Cdc42fl/fl mice are presented. (F) Bone marrow cells were cultured, and cell survival was determined at days 1 to 3. Percent survival was calculated as the percentage of viable neutrophil numbers determined by counting viable cells and FACS analysis of live neutrophils (7-AAD-negative CD11b+ Gr1+ cells) at each day relative to the initial cell numbers. Shown is a representative result of two independent experiments.