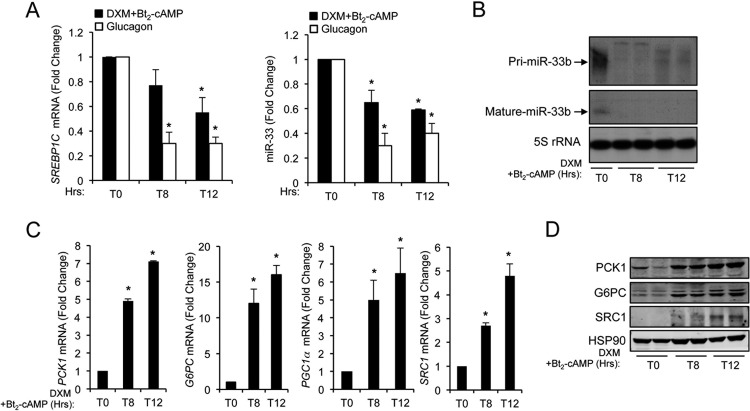
Fig 1.
Induction of glucose synthesis reduces SREBP1 and miR-33b expression levels. (A) qRT-PCR analysis of SREBP1c mRNA (left) and miR-33b (right) expression levels in HepG2 cells cultured in glucose-free medium in the presence of lactate-pyruvate (2 and 20 mM, respectively) and treated with dexamethasone (DXM) and 2′-O-dibutyryladenosine 3′-5′-monophosphate (Bt2-cAMP) or glucagon during 8 and 12 h. Data are presented as means ± SEM from 3 independent experiments performed in triplicate. ∗, P ≤ 0.05 versus untreated cells (T0). (B) Northern blot analysis of pri-miR-33b (precursor form) and miR-33b (mature form) in HepG2 cells cultured in glucose-free medium in the presence of lactate-pyruvate (2 and 20 mM, respectively) and treated with DXM and Bt2-cAMP during 8 and 12 h. 5S rRNA was used as a loading control. (C) qRT-PCR analysis of PCK1, G6PC, PGC1α, and SRC1 expression levels in HepG2 cells cultured in glucose-free medium in the presence of lactate-pyruvate (2 and 20 mM, respectively) and treated with DXM and Bt2-cAMP during 8 and 12 h. Data are presented as means ± SEM from 3 independent experiments performed in triplicate. ∗, P ≤ 0.05 versus nontreated cells (T0). (D) Representative Western blot analysis of SRC1, G6PC, and PCK1 expression levels in HepG2 cells cultured in glucose-free medium in the presence of lactate-pyruvate (2 and 20 mM, respectively) and treated with DXM and Bt2-cAMP during 8 and 12 h. HSP90 was used as a loading control.