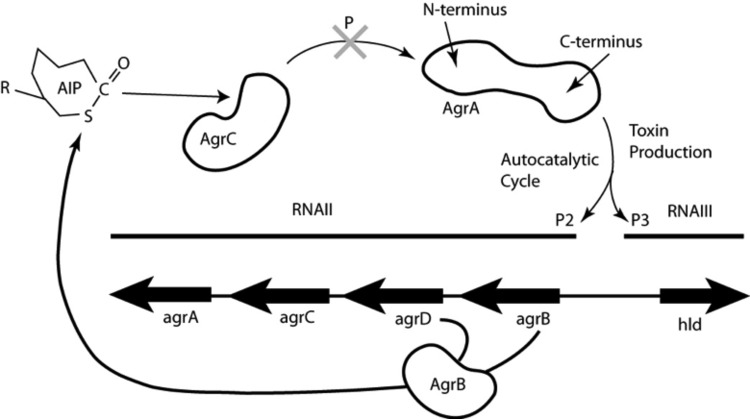
Fig 1.
S. aureus agr operon for toxin production. The cyclic autoinducing peptide (AIP) is the signaling molecule coded for by agrD and processed by AgrB. Mature AIP is secreted to the cell surface, where it binds to and activates the histidine kinase AgrC on the same cell or on a different cell. Subsequently, AgrC autophosphorylates and transfers its phosphoryl group to Asp 59 on the N-terminal domain of the response regulator protein AgrA. Phosphorylated AgrA undergoes a conformational change to form a dimer, which enables its C-terminal DNA-binding domains to bind to promoter P2 to activate AIP transcription in an autocatalytic fashion. When the AIP concentration reaches a certain threshold, AgrA also binds to the 10-fold-weaker promoter P3, which drives the transcription of RNAIII, a master regulator of expression of a series of toxins and virulence factors in the post-exponential-growth phase (6). RNAIII encodes the toxin delta-hemolysin (Hld). The drug discovery target in this work is the inhibition of phosphoryl transfer to AgrA, as indicated by the large shaded X at the top center.