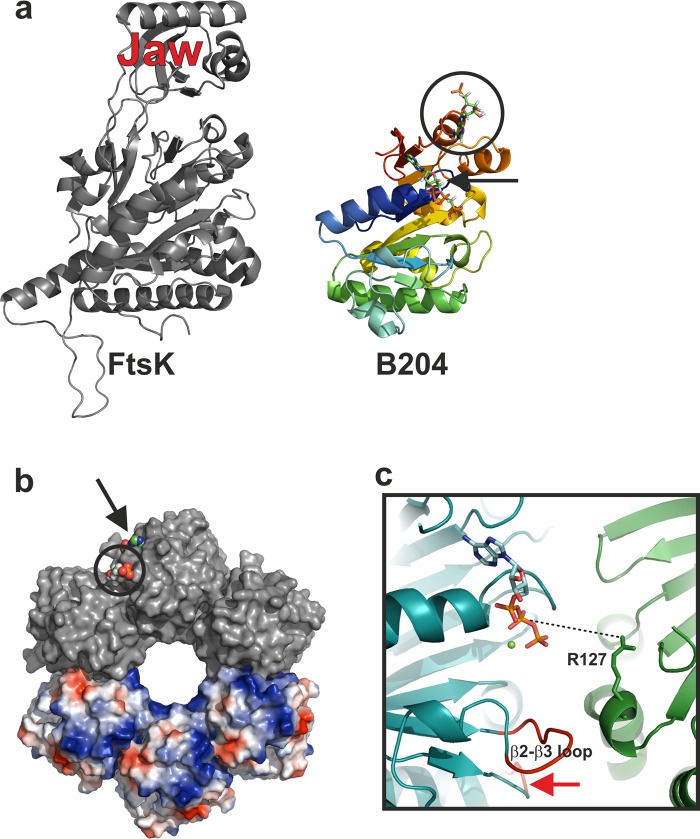
Fig 6.
Comparison of FtsK and B204 and suggested multimeric form of B204. (a) Comparison of FtsK and B204. FtsK (PDB accession number 2iuu) is colored in gray and B204 in rainbow hues (blue to red). The AMPPCP bound to B204 is shown for clarity and indicated with a black arrow. The additional nucleotide bound to B204 is highlighted with a black circle. The FtsK jaw domain is indicated. (b) A hexamer model of B204 with three subunits (top) surface rendered in gray and three subunits (bottom) rendered as electrostatic surfaces. The B204 active site is indicated with a black arrow and the additional nucleotide-binding site with a black circle; bound AMPPCP nucleotides are shown as space-filling models. (c) Close-up of the nucleotide-binding site. A dotted line joins the arginine finger (R127) that should reach into the active site from the adjacent monomer to hydrogen bond with the β-phosphate of AMPPCP. The β2-β3 loop is labeled, and the β4-α3 loop is indicated with a red arrow.