Abstract
OBJECTIVE
We sought to examine the association of labor induction and perinatal outcomes.
STUDY DESIGN
This was a retrospective cohort study of low-risk nulliparous women with term, live births. Women who had induction at a given gestational age (eg, 39 weeks) were compared to delivery at a later gestation (eg, 40, 41, or 42 weeks).
RESULTS
Compared to delivery at a later gestational age, those induced at 39 weeks had a lower risk of cesarean (adjusted odds ratio [aOR], 0.90; 95% confidence interval [CI], 0.88–0.91) and labor dystocia (aOR, 0.88; 95% CI, 0.84–0.94). Their neonates had lowered risk of having 5-minute Apgar <7 (aOR, 0.81; 95% CI, 0.72–0.92), meconium aspiration syndrome (aOR, 0.30; 95% CI, 0.19–0.48), and admission to neonatal intensive care unit (aOR, 0.87; 95% CI, 0.78–0.97). Similar findings were seen for women who were induced at 40 weeks compared to delivery later.
CONCLUSION
Induction of labor in low-risk women at term is not associated with increased risk of cesarean delivery compared to delivery later.
Keywords: cesarean, induction, neonatal outcomes
Induction of labor is among the most common obstetric interventions. In 2008, 23.1 per 100 (23.1%) live births in the United States had labor induction,1 and this represents more than a doubling of the frequency in the 1990s.2 The goal of induction of labor is to achieve a vaginal delivery when the benefits of expeditious delivery outweigh the potential risk of continuing pregnancy.3 While indications of labor induction are not definitive, the most common indication for induction is postterm pregnancy, which is known to carry increased risk of perinatal mortality, meconium aspiration, and intrauterine infection for the neonate as well as increased risk of perineal trauma, labor dystocia, and cesarean delivery for the mother.4 As perinatal morbidity increases in a continuous, not threshold, fashion >39 weeks’ gestation,5 it is unclear whether induction of labor <42 weeks in low-risk pregnancy may improve perinatal outcomes.
The prevailing belief that induction of labor increases the risk of cesarean delivery likely stems from observational studies that compared women who had induction of labor to women with spontaneous labor at a particular gestational age (GA).6-9 This association has not been validated by prospective trials. A systematic review of existing literature identified 9 randomized controlled trials that report an overall decreased risk of cesarean in women who were induced in comparison to those who were expectantly managed, particularly at GA ≥41 weeks10-12; evidence is less clear <41 weeks.13 The discrepancies in findings between observational studies and prospective trials likely resides in that a pregnant woman can either undergo induction of labor or she can continue pregnancy and deliver later; her options are not induction of labor now or spontaneous labor now. Thus, the appropriate comparator (ie, counterfactual) for women who undergo labor induction at a given GA (eg, 39 weeks) is women who do not have labor induction at that GA (ie, who are expectantly managed) and subsequently deliver at a later GA (eg, 40, 41, or 42 weeks). Yet, even when labor inductions were compared to expectant management in recent observational studies, such data remain conflicting.14-16 Further, since detrimental neonatal morbidities are very rare events, existing data may not have sufficient statistical power to evaluate differences in neonatal outcomes and risks/benefits of induction vs expectant management.13,17,18
Given this background, we aimed to examine the association between induction of labor and cesarean delivery and associated perinatal outcomes. We analyzed a large population-based cohort of nulliparous women who had singleton live births in the United States in 2005. Specifically, we compared low-risk women who had induction of labor at a given GA to women who delivered at a later GA.
Materials and Methods
This is a retrospective study of maternal and infant data from live births delivered in the United States in 2005, using the Vital Statistics Natality birth certificate registry provided by the Centers for Disease Control and Prevention. This dataset includes all live births to US and non-US residents occurring in the 50 United States, the District of Columbia, the Virgin Islands, and US territories. The 2005 birth certificate data could be collected by using the 2003 version of the US Standard Certificate of Birth (used by 12 states and representing 31% of births: Florida, Idaho, Kansas, Kentucky, Nebraska, New Hampshire, New York, Pennsylvania, South Carolina, Tennessee, Texas, Washington, and Puerto Rico) or the 1989 version of the US standard certificate of birth (used by the remainder of the states, the District of Columbia, and territories).19 This analysis only included births from the 12 states that used the 2003 version of US Standard Certificate of Birth. While most information remained the same or compatible between the 2 revisions, some outcome recordings were different.
The target study population was nulliparous women with singleton, vertex live births delivered at 39-42 weeks’ gestation. In both versions of the US standard certificate of birth, GA was recorded as 2 variables, 1 based on obstetric/clinical estimation and 1 based on last menstrual period (LMP). Because accurate determination of GA is paramount to this study analysis, only births with the same GA by both LMP and clinical/obstetric dating were included in the study cohort. GA at delivery was categorized as 39, 40, 41, or 42 completed weeks. Women with prior live births or total births (including index pregnancy) >1 were considered multiparous and excluded, as were those who did not have information on whether labor was induced or spontaneous. Other exclusion criteria were: multifetal gestations, non-vertex presentation, and delivery <39 weeks’ or >42 weeks’ gestation. To identify a low-risk cohort of women, we excluded women with chronic hypertension, gestational or pregestational diabetes mellitus, or placenta previa. Additionally, women with gestational hypertension or preeclampsia/eclampsia, and oligohydramnios/polyhydramnios were also excluded from the induction group as we sought to identify women undergoing elective induction, but allowed these women to remain in the expectant management group as development of these late complications of pregnancy is a risk with expectant management. The diagnostic criteria of conditions and outcomes were based on definitions compiled by a committee of federal and state health statistics officials for the Association of Vital Record and Health Statistics.20 The National Center for Health Statistics regulates the birth certificate information, checking it for completeness, validity, and consistency between items. Data collection and coding process are reviewed on an ongoing basis for quality control.21
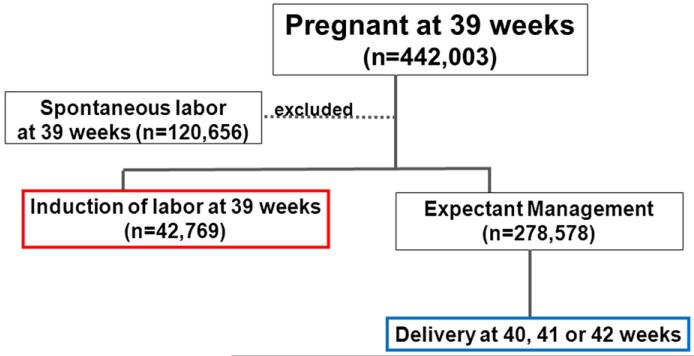
To examine the association between induction of labor and perinatal outcomes, we compared low-risk nulliparous women who had induction of labor at a given GA (eg, 39 weeks) to women who continued pregnancy with delivery at 40, 41, or 42 weeks by either spontaneous labor or induction of labor. To illustrate, for pregnancies at 39 weeks, we excluded those who went into spontaneous labor at 39 weeks (Figure); we assigned low-risk pregnancies at 39 weeks that were induced as the “induction” group; we assigned pregnancies that delivered at a later gestation (40, 41, or 42 weeks) to the “expectant” group (Figure). Of note, pregnancies designated as the expectant group could undergo either spontaneous labor or induction of labor at a later GA if conditions that necessitated induction arose late. We made a similar analytic scheme for women who had induction of labor at 40 weeks and compared them to women who delivered at a later GA (41 or 42 weeks); women who had induction at 41 weeks were compared to deliveries at 42 weeks. Ideally, we could have compared women who were induced at 39 weeks 0 days to those who delivered at 39 weeks 1 day, and then compared women who were induced at 39 weeks 1 day to those delivered at 39 weeks 2 days–1 day and 1 decision point at a time–to discern the benefits/risks of induction compared to expectant management. In reality, clinical decisions often are made with each week of gestation as the unit; and in the Vital Statistics Natality dataset, GA was recorded in weeks rather than days as the unit. Thus, we compared women who were induced at 39 weeks to those with ongoing pregnancy at 40 weeks, with the intention of not including women who went into spontaneous labor at 39 weeks, so as to avoid the fallacy of comparing induction of labor to spontaneous labor.
FIGURE.
Flow diagram of study groups comparing induction of labor at a given GA to delivery at a later GA
GA, gestational age.
The primary outcome was the frequency of cesarean delivery and operative vaginal delivery (including vacuum-assisted vaginal delivery and/or forceps delivery). Secondary outcomes included 5-minute Apgar score <7, neonatal injury (in vaginal deliveries only), meconium aspiration, and “neonatal morbidity” as a composite variable of 5-minute Apgar <7, meconium aspiration syndrome, birth injury, use of mechanical ventilation >30 minutes or >6 hours, neonatal antibiotics use, neonatal seizure, and admissions to the neonatal intensive care unit (NICU). Any neonate with >1 of the above conditions/diagnoses was only counted once as having neonatal morbidity. Again, maternal and neonatal outcomes were collected and reported using either the 1989 or the 2003 Revision of Standard Certificate of Birth. Perinatal outcomes were examined at 39, 40, and 41 weeks and compared to delivery at a later GA using χ2 test. Multivariable logistic regression analyses were used to control for confounding bias. Covariates included in the regression model were: maternal age, race/ethnicity, education, gestational weight gain, number of prenatal visits, and cigarette smoking. Additionally, to account for women with longer gestation who tend to have higher weight gain, we coded weight gain as average gestational weight gain per week. Statistical analysis was performed using software (STATA, version 11.0; StataCorp, College Station, TX). Statistical significance was indicated by P value < .05 or 95% confidence intervals (CIs). Institutional review board approval was obtained from the Committee on Human Research at the University of California, San Francisco.
Results
There were 442,003 low-risk nulliparous women who met study inclusion criteria. The majority of women were between the age of 20-34 years (73.3%), of non-Hispanic white race/ethnicity (62.5%), had >8 years of education (80.8%), had gestational weight gain <35 lb (58.2%), and has at least 8 prenatal care visits (89.8%) (Table 1).
TABLE 1.
Maternal characteristics (total n = 442,003)
| Characteristic | No. of women | % |
|---|---|---|
| Age, y | ||
| ≤19 | 85,752 | 19.4 |
| 20-34 | 323,846 | 73.3 |
| ≥35 | 32,403 | 7.3 |
| Race/ethnicity | ||
| Non-Hispanic white | 276,350 | 62.5 |
| African American | 58,880 | 13.3 |
| Latina/Hispanic | 71,957 | 16.3 |
| Asian | 24,153 | 5.5 |
| Other | 10,683 | 2.4 |
| Education, y | ||
| 0-8 (<high school) | 80,307 | 18.1 |
| 9-12 (some high school/graduate) | 116,091 | 26.3 |
| 13 to ≥16 (some college/≥graduate) | 240,650 | 54.5 |
| Not stated/unknown | 4953 | 1.1 |
| Gestational weight gain, lb | ||
| ≤35 | 278,404 | 58.2 |
| >35 | 199,213 | 41.7 |
| Prenatal care visits | ||
| <8 | 43,070 | 10.2 |
| ≥8 | 377,918 | 89.8 |
| Marital status | ||
| Not married | 186,307 | 42.2 |
| Married | 255,696 | 57.8 |
| Gestational age at delivery, wk | ||
| 39 | 181,328 | 41.0 |
| 40 | 190,578 | 43.1 |
| 41 | 65,831 | 14.9 |
| 42 | 4266 | 1.0 |
Source: National Center for Health Statistics (2005).19
Comparing women who had induction at 39 weeks’ GA to women delivered at a later GA (40, 41, or 42 weeks), the frequency of cesarean was 26.2% among those who had induction and 28.4% for women who delivered at a later GA (by either labor induction or spontaneous labor) (Table 2). The association between induction and cesarean delivery was examined using multivariable logistic regression models to control for potential confounders. Compared to delivery later, induction of labor was associated with lower odds of cesarean (adjusted odds ratio [aOR], 0.90; 95% CI, 0.88–0.91) (Table 2). Similarly, induction at 40 weeks was associated with a lower risk of cesarean delivery (aOR, 0.88; 95% CI, 0.86–0.92) as was induction at 41 weeks compared to delivery later (Table 2). There was no difference in risk of operative vaginal delivery (including forceps or vacuum-assisted vaginal delivery) in women who either had induction at 39 weeks or delivery later (14.3% vs 12.9%, respectively; aOR, 1.11; 95% CI, 0.97–1.15). For those who did have induction at 40 weeks, and who had induction at 41 weeks, they had a higher risk of having an operative vaginal delivery compared to delivery later (Table 2). We also examined the occurrence of fetal macrosomia (defined as birthweight >4000 g regardless of GA). In women who underwent induction at 39 weeks, the frequency of macrosomia was 6.4% while it was 11.9% for those delivered at a later gestation (aOR, 0.33; 95% CI, 0.30–0.35). The risk of macrosomia was similarly decreased for induction at 40 weeks and at 41 weeks compared to delivery later (Table 2).
TABLE 2.
Frequency and adjusted ORs of mode of delivery and birthweight at time of induction compared to delivery at later GA
| Variable | aORa | 95% CI | |
|---|---|---|---|
| Cesarean delivery | |||
| 39 wk’ GA | |||
| Induction (n = 42,769) | 26.2% | 0.90 | 0.88–0.91 |
| Expectant (n = 278,578) | 28.4% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 52,383) | 31.0% | 0.88 | 0.86–0.92 |
| Expectant (n = 74,860) | 33.7% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 28,325) | 36.0% | 0.89 | 0.83–0.95 |
| Expectant (n = 4744) | 39.0% | Referent | |
| Operative vaginal deliveryb | |||
| 39 wk’ GA | |||
| Induction (n = 31,574) | 14.3% | 1.11 | 0.97–1.15 |
| Expectant (n = 199,390) | 12.9% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 36,129) | 15.5% | 1.16 | 1.12–1.22 |
| Expectant (n = 49,628) | 13.4% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 18,044) | 15.7% | 1.30 | 1.15–1.47 |
| Expectant (n = 2893) | 12.0% | Referent | |
| Birthweight >4000 g | |||
| 39 wk’ GA | |||
| Induction (n = 42,947) | 6.4% | 0.33 | 0.30–0.35 |
| Expectant (n = 279,733) | 11.9% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 56,606) | 11.0% | 0.62 | 0.59–0.64 |
| Expectant (n = 75,224) | 16.5% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 28,470) | 16.7% | 0.75 | 0.68–0.81 |
| Expectant (n = 4772) | 20.2% | Referent |
aOR, adjusted odds ratio; CI, confidence interval; GA, gestational age.
Multivariable logistic regression controlling for: maternal age, race/ethnicity, number of prenatal care visits, gestational weight gain, cigarette smoking during pregnancy, education; referent comparison group composed of women who did not have induction of labor (ie, expectantly managed) and delivered at later GA
Operative vaginal delivery examined among women who did not have cesarean deliveries as denominator.
Source: National Center for Health Statistics (2005).19
Next, we examined labor characteristics associated with induction/expectant management. As expected based on the association between fetal macrosomia and labor dystocia, our data showed that the risk of labor dystocia for women who were induced at 39 weeks (5.93%) was lower than for those expectantly managed and delivered later (6.71%; aOR, 0.88; 95% CI, 0.84–0.94). Labor dystocia was also less likely for women who had induction at 40 weeks compared to delivery later (Table 3). The diagnosis of fetal intolerance of labor was less frequent in women who had induction at 39 weeks (6.15%) compared to delivery later (7.12%; aOR, 0.86; 95% CI, 0.81–0.92), while no statistically significant differences were seen for 40- or 41-week comparisons (Table 3). Additionally, there was a protective association between induction and chorioamnionitis, with decreased risk for induction at 39 weeks compared to delivery later and induction at 40 weeks compared to delivery later (Table 3).
TABLE 3.
Frequency and adjusted ORs of conditions associated with labor/delivery at time of induction compared to delivery at later GA
| Variable | aORa | 95% CI | |
|---|---|---|---|
| Labor dystocia | |||
| 39 wk’ GA | |||
| Induction (n = 24,006) | 5.93% | 0.88 | 0.84–0.94 |
| Expectant (n = 178,413) | 6.71% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 30,331) | 7.21% | 0.81 | 0.78–0.86 |
| Expectant (n = 48,727) | 8.81% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 17,450) | 9.82% | 0.94 | 0.64–2.22 |
| Expectant (n = 2746) | 12.4% | Referent | |
| Fetal intolerance of labor | |||
| 39 wk’ GA | |||
| Induction (n = 24,006) | 6.15% | 0.86 | 0.81–0.92 |
| Expectant (n = 178,413) | 7.12% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 30,331) | 8.00% | 0.99 | 0.93–1.04 |
| Expectant (n = 48,727) | 8.18% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 17,450) | 9.12% | 1.15 | 0.99–1.33 |
| Expectant (n = 2746) | 8.41% | Referent | |
| Chorioamnionitis | |||
| 39 wk’ GA | |||
| Induction (n = 42,936) | 2.52% | 0.71 | 0.67–0.77 |
| Expectant (n = 279,706) | 3.54% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 52,606) | 3.20% | 0.78 | 0.73–0.83 |
| Expectant (n = 75,218) | 4.13% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 28,470) | 4.45% | 1.08 | 0.96–1.27 |
| Expectant (n = 4772) | 4.30% | Referent |
aOR, adjusted odds ratio; CI. confidence interval; GA, gestational age.
Multivariable logistic regression controlling for: maternal age, race/ethnicity, number of prenatal care visits, gestational weight gain, cigarette smoking during pregnancy, education; referent comparison group composed of women who did not have induction of labor (ie, expectantly managed) and delivered at later GA.
Source: National Center for Health Statistics (2005).19
Next, we examined neonatal outcomes associated with induction compared to expectant management. We observed a lower risk of 5-minute Apgar score <7 in women who had induction compared to expectant management–this association was seen for induction at 39, 40, and 41 weeks compared to the expectant groups (Table 4). The adjusted odds of meconium aspiration was statistically significantly lower for induction at 39 weeks compared to expectant management (aOR, 0.30; 95% CI, 0.19–0.48); it was also lower at 40 weeks (aOR, 0.57; 95% CI, 0.43–0.78) (Table 4). Similar associations between induction and mechanical ventilator use for >6 hours as well as NICU admission were seen for the 39- and 40-week thresholds but not for the 41-week threshold (Table 4). Given the rarity of neonatal complications, we created a composite neonatal morbidity variable that included all births with ≥1 of the following: 5-minute Apgar score <7, meconium aspiration syndrome, ventilator use >30 minutes or >6 hours, birth injury, neonatal seizure, neonatal antibiotics use, and admissions to the NICU. Again, we observed that induction of labor, compared to expectant management, is associated with decreased risk of composite morbidity at the 39-week threshold (2.55% for induction, 2.97% for expectant; aOR, 0.87; 95% CI, 0.81–0.93), at the 40-week threshold (2.74% for induction, 3.50% for expectant; aOR, 0.79; 95% CI, 0.74–0.85), and at the 41-week threshold compared to deliveries at 42 weeks (Table 4).
TABLE 4.
Frequency and adjusted ORs of neonatal outcomes at time of induction compared to delivery at later GA
| Variable | aORa | 95% CI | |
|---|---|---|---|
| 5 min Apgar <7 | |||
| 39 wk’ GA | |||
| Induction (n = 42,793) | 0.89% | 0.81 | 0.72–0.92 |
| Expectant (n = 278,612) | 1.09% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 52,469) | 1.00% | 0.80 | 0.71–0.90 |
| Expectant (n = 74,952) | 1.27% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 28,381) | 1.19% | 0.66 | 0.51–0.86 |
| Expectant (n = 4729) | 1 78% | Referent | |
| Meconium aspiration | |||
| 39 wk’ GA | |||
| Induction (n = 23,963) | 0.08% | 0.30 | 0.19–0.48 |
| Expectant (n = 177,733) | 0.29% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 30,263) | 0.20% | 0.57 | 0.43–0.78 |
| Expectant (n = 48,518) | 0.39% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 17,379) | 0.33% | 0.92 | 0.45–1.89 |
| Expectant (n = 2739) | 0.40% | Referent | |
| Ventilator use >6 h | |||
| 39 wk’ GA | |||
| Induction (n = 18,890) | 0.25% | 0.68 | 0.49–0.94 |
| Expectant (n = 100,892) | 0.36% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 22,194) | 0.28% | 0.56 | 0.40–0.78 |
| Expectant (n = 26,364) | 0.47% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 10,980) | 0.56% | 1.58 | 0.67–3.70 |
| Expectant (n = 2003) | 0.35% | Referent | |
| NICU admission | |||
| 39 wk’ GA | |||
| Induction (n = 18,890 | 2.57% | 0.87 | 0.78–0.97 |
| Expectant (n = 100,892) | 3.05% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 22,194) | 2.70% | 0.74 | 0.66–0.83 |
| Expectant (n = 26,364) | 3.60% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 10,980) | 3.48% | 0.97 | 0.72–1.30 |
| Expectant (n = 2003) | 4.04% | Referent | |
| Composite morbidityb | |||
| 39 wk’ GA | |||
| Induction (n = 42,853) | 2.55% | 0.87 | 0.81–0.93 |
| Expectant (n = 278,625) | 2.97% | Referent | |
| 40 wk’ GA | |||
| Induction (n = 52,457) | 2.74% | 0.79 | 0.74–0.85 |
| Expectant (n = 74,882) | 3.50% | Referent | |
| 41 wk’ GA | |||
| Induction (n = 28,359) | 3.33% | 0.69 | 0.59–0.81 |
| Expectant (n = 4742) | 4.90% | Referent |
aOR, adjusted odds ratio; CI, confidence interval; GA, gestational age; NICU, neonatal intensive care unit.
Multivariable logistic regression controlling for: maternal age, race/ethnicity, number of prenatal care visits, gestational weight gain, cigarette smoking during pregnancy, education; referent comparison group composed of women induced at specific GA (39, 40, or 41 wk)
Includes: 5-min Apgar score <7, meconium aspiration syndrome, ventilator use >30 min or >6 h, birth injury, neonatal seizure, neonatal antibiotics use, and NICU admission.
Source: National Center for Health Statistics (2005).19
Comment
In this large cohort of nulliparous women who delivered singleton live births between 39-42 weeks, we observed that induction of labor was not associated with an increased risk of cesarean delivery compared to delivery at a later GA. While a majority of previous observational studies compared women who had induction of labor to spontaneous labor at similar GA and reported increased risk of cesarean, our analytic scheme more accurately reflects the clinical management options: either to undergo induction now or continuing pregnancy, leading to delivery at a later GA–either by entering spontaneous labor or induction of labor for other indications.10-12,22 With such comparisons, our findings are consistent with randomized, prospective studies that have examined induction of labor vs expectant management.13 Additionally, we examined neonatal outcomes between induction and expectant management at 39-, 40-, or 41-week threshold. While it appears that induction of labor at 39 or 40 weeks may offer potential benefits, we are not advocating changing clinical practice or recommending offering induction of labor for low-risk women at 39 or 40 weeks. Further evidence from large, prospective studies is needed to elucidate and validate whether intervention may lead to reduced risk of cesarean delivery and improved neonatal outcome.
It appeared that the potential benefit of induction, compared to expectant management, for both maternal and neonatal outcomes was more consistently significant for 39- and 40-week GA thresholds but not always significant at the 41-week induction vs 42-week delivery level. One explanation may be that it has become a common practice for clinicians to offer induction of labor at 41 weeks’ gestation such that delivery at ≥42 weeks is less common4,5,23; therefore, we may have lacked statistical power to detect a difference. Alternatively, it could be that pregnancies that progressed >40 or 41 weeks almost always undergo close antenatal surveillance such that these pregnancies truly might be at a lower risk of perinatal complications as those at risk identified by antenatal monitoring would have been delivered at an earlier GA. While many of our effect estimates appeared small in magnitude (eg, a 0.9 reduction in adjusted odds of cesarean with induction at 39 weeks compared to expectant management), at a population level, a small difference between groups can still represent a large impact. While we certainly do not advocate clinicians offering elective induction of labor at 39 weeks to low-risk women, our study findings suggest that there may be potential benefits that can be clinically significant as well as statistically significant such that this clinical question deserves further investigation.
Management of pregnancy relies on accurate determination of GA, and dating based on LMP has been shown to be less reliable than obstetric (sonographic) dates.24,25 For this study, we limited the analysis to only women whose reported GA was concordant by both obstetric/sonographic dating and by LMP dating. This inclusion criterion likely yielded a cohort that has well-established dates. With such inclusion criteria, we observed that the risk of hyaline membrane disease was no different between the induction and expectant management groups at 39 weeks. This observation supports the American Congress of Obstetricians and Gynecologists recommendation that “the gestational age of the fetus should be determined to be at least 39 weeks or that fetal lung maturity must be established before induction.”1 While hyaline membrane disease is associated with prematurity, meconium aspiration syndrome is often considered a morbidity subsequent to dysmaturity. We observed that the risk of mechanical ventilator support, as was meconium aspiration syndrome, was lower for neonates following induction at 40 and 41 weeks compared to expectant management. Yet, we emphasize that our data are insufficient to recommend change in clinical practice; rather, large prospective studies are necessary to further examine the relationship between induction of labor at term and maternal/neonatal morbidities.
This study is one of the largest to date to examine the association between induction/expectant management and mode of delivery/neonatal outcomes, but it has limitations. The retrospective cohort design of the study is susceptible to confounding. While we used multivariable logistic regression models to control for potential confounders, variables collected by the birth certificate data likely did not capture complete confounding such that there might be residual confounding due to unmeasured or unobserved confounders. This population-based study aimed to examine a low-risk population. Although we attempted to exclude most women with obstetric or medical conditions complicating pregnancy, there may be some high-risk pregnancies that remained in the study population. Given that women with medical/obstetric conditions are more likely to undergo induction and they are also more likely to have cesarean deliveries, the incorporation of these high-risk women in the induction group may result in higher estimates of cesarean in the induction group, biasing our estimates toward the null. Additionally, detailed obstetrical information such as precise indication of induction or cervical examination on admission was not available. We acknowledge that cervical status may influence a clinician’s assessment of risk/benefits of induction of labor. Recent studies suggests that elective induction of labor (compared to expectant management) increased the utilization of labor and delivery resources but did not differ in perinatal outcomes among nulliparous women with an unfavorable cervix15 as well as those with favorable cervical status.14 Another important aspect of labor management is women’s perception, preference, and experience regarding the birth of their children. This study was not able to assess the impact of cost or patient preference/satisfaction, 2 important issues when considering labor induction. Women with spontaneous labor report the highest level of satisfaction with their experience, and women undergoing induction are more likely to report dissatisfaction with the labor process.26 Providing patient-centered, evidence-based care necessitates understanding the patient’s needs and values in addition to assessing perinatal outcomes associated with induction of labor.
In summary, our retrospective study examines whether induction at 39, 40, or 41 weeks’ gestation compared to expectant management may be associated with decreased risk of cesarean delivery. While our study supports that induction may provide improved perinatal outcomes, future large prospective, randomized, clinical trials are necessary to further assess the potential benefit in the low-risk population in such a clinical setting.
Acknowledgments
Y.W.C. is supported by the University of California San Francisco Women’s Reproductive Health Research Career Development Award, National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD001262).
Footnotes
The authors report no conflict of interest.
Contributor Information
Yvonne W. Cheng, Division of Maternal-Fetal Medicine, Department of Obstetrics, Gynecology, and Reproductive Medicine, University of California, San Francisco, School of Medicine, San Francisco, CA.
Anjali J. Kaimal, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA.
Jonathan M. Snowden, Department of Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR.
James M. Nicholson, Department of Family and Community Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA.
Aaron B. Caughey, Department of Obstetrics and Gynecology, Oregon Health and Science University, Portland, OR.
REFERENCES
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJK. Births: final data for 2008. Natl Vital Stat Rep. 2010;59:1–72. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2006. Natl Vital Stat Rep. 2009;57:1–102. [PubMed] [Google Scholar]
- 3.ACOG Committee on Practice Bulletins-Obstetrics American College of Obstetricians and Gynecologists practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114:386–97. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics ACOG practice bulletin no. 55 (replaces practice pattern no. 6, October 1997): management of postterm pregnancy. Obstet Gynecol. 2004;104:639–46. doi: 10.1097/00006250-200409000-00052. [DOI] [PubMed] [Google Scholar]
- 5.Caughey AB, Washington AE, Laros RK., Jr. Neonatal complications of term pregnancy: rates by gestational age increase in a continuous, not threshold, fashion. Am J Obstet Gynecol. 2005;192:185–90. doi: 10.1016/j.ajog.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 6.Yeast JD, Jones A, Poskin M. Induction of labor and the relationship to cesarean delivery: a review of 7001 consecutive inductions. Am J Obstet Gynecol. 1999;180:628–33. doi: 10.1016/s0002-9378(99)70265-6. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DP, Davis RN, Brown AJ. Risk of cesarean delivery after induction at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol. 2003;188:1565–9. doi: 10.1067/mob.2003.458. [DOI] [PubMed] [Google Scholar]
- 8.Vrouenraets FP, Roumen FJ, Dehinq CJ, van den Akker ES, Arts MJ, Scheve EJ. Bishop score and risk of cesarean delivery after induction in nulliparous women. Obstet Gynecol. 2005;105:690–7. doi: 10.1097/01.AOG.0000152338.76759.38. [DOI] [PubMed] [Google Scholar]
- 9.Vahratian A, Zhang J, Troendel JF, Sciscione AC, Hoffman MK. Labor progression and risk of cesarean delivery in electively induced nulliparas. Obstet Gynecol. 2005;105:698–704. doi: 10.1097/01.AOG.0000157436.68847.3b. [DOI] [PubMed] [Google Scholar]
- 10.The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units A clinical trial of induction of labor versus expectant management in postterm pregnancy. Am J Obstet Gynecol. 1994;170:716–23. [PubMed] [Google Scholar]
- 11.Chanrachakul B, Herabutya Y. Postterm with favorable cervix: is induction necessary? Eur J Obstet Gynecol Reprod Biol. 2003;106:154–7. doi: 10.1016/s0301-2115(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 12.Hannah ME, Hannah WJ, Hellmann J, Hewson S, Milner R, Willan A, The Canadian Multicenter Post-term Pregnancy Trial Group Induction of labor as compared with serial antenatal monitoring in post-term pregnancy: a randomized controlled trial. N Engl J Med. 1992;326:1587–92. doi: 10.1056/NEJM199206113262402. [DOI] [PubMed] [Google Scholar]
- 13.Caughey AB, Sundaram V, Kaimal AJ, et al. Maternal and neonatal outcomes of elective induction of labor. Evid Rep Technol Assess. 2009;176:1–257. [PMC free article] [PubMed] [Google Scholar]
- 14.Osmundson SS, Ou-Yang RJ, Grobman WA. Elective induction compared with expectant management in nulliparous women with a favorable cervix. Obstet Gynecol. 2010;116:601–5. doi: 10.1097/AOG.0b013e3181eb6e9b. [DOI] [PubMed] [Google Scholar]
- 15.Osmundson SS, Ou-Yang RJ, Grobman WA. Elective induction compared with expectant management in nulliparous women with an unfavorable cervix. Obstet Gynecol. 2011;117:583–7. doi: 10.1097/AOG.0b013e31820caf12. [DOI] [PubMed] [Google Scholar]
- 16.Glantz JC. Term labor induction compared with expectant management. Obstet Gynecol. 2010;115:70–6. doi: 10.1097/AOG.0b013e3181c4ef96. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Ramos L, Olivier F, Delke I, Kaunitz AM. Labor induction versus expectant management for postterm pregnancies: a systematic review with meta-analysis. Obstet Gynecol. 2003;101:1312–8. doi: 10.1016/s0029-7844(03)00342-9. [DOI] [PubMed] [Google Scholar]
- 18.Hannah ME, Huh C, Hewson SA, Hannah WJ. Postterm pregnancy: putting the merits of a policy of induction of labor into perspective. Birth. 1996;23:13–9. doi: 10.1111/j.1523-536x.1996.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Martin JA, Hamilton BE, Sutton PD, et al. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System [Accessed Oct. 8, 2012];Births: final data for 2005. Natl Vital Stat Rep. 2007 56:1–103. Available at: http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm. [PubMed] [Google Scholar]
- 20.National Center for Health Statistics . Guide to completing the facility worksheets for the certificate of live birth and report of fetal death (2003 revision) National Center for Health Statistics; Hyattsville, MD: [Accessed March 22, 2012]. Available at: www.cdc.gov/nchs/data/dvs/guidetocompletefacilitywks.pdf. [Google Scholar]
- 21.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54:1–116. [PubMed] [Google Scholar]
- 22.Caughey AB, Nicholson JM, Cheng YW, Lyell DJ, Washington AE. Induction of labor and cesarean delivery by gestational age. Am J Obstet Gynecol. 2006;195:700–5. doi: 10.1016/j.ajog.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Gulmezoglu AM, Crowther CA, Middleton P. Induction of labor for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev. 2006;4:CD004945. doi: 10.1002/14651858.CD004945.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California live birth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(Suppl):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007;21(Suppl):86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 26.Shetty A, Burt R, Rice P, Templeton A. Women’s perceptions, expectations and satisfaction with induced labor–a questionnaire-based study. Eur J Obstet Gynecol Reprod Biol. 2005;123:56–61. doi: 10.1016/j.ejogrb.2005.03.004. [DOI] [PubMed] [Google Scholar]