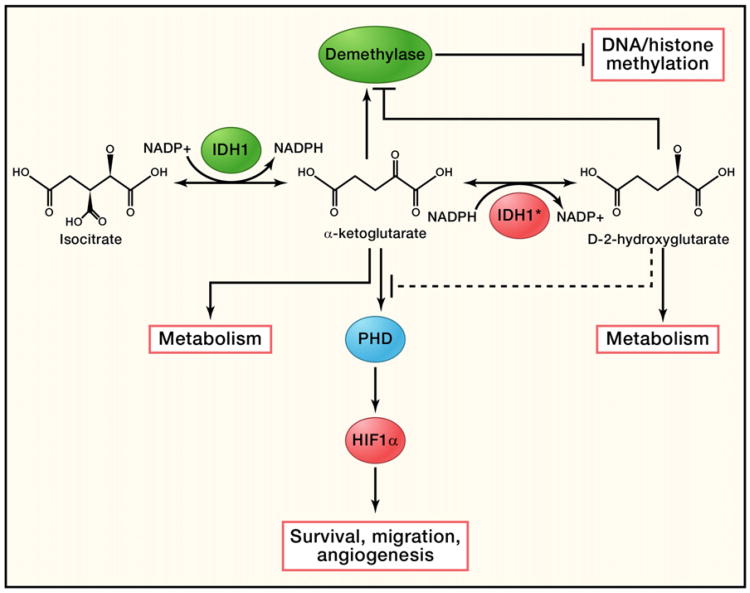
Figure 2. Function of Normal and Mutated IDH1.
Wild-type IDH1 catalyzes isocitrate to form α-ketoglutarate and convert NADP+ to NADPH at the same time. A mutated form of IDH1 can convert α-ketoglutarate to D2-hydroxyglutarate in an NADPH-dependent manner. Excessive D2-hy-droxyglutarate results in cellular stress as well as metabolic changes. It could also potentially act as a competitive substrate to inhibit DNA/histone methyltransferases and prolyl hydroxylases (PHDs), resulting in DNA/histone hypomethylation or activation of HIF-1α, which can be further accelerated by the lack of α-ketoglutarate, as α-ketoglutarate is a key substrate for both PHDs and DNA/histone methyltransferases.