Figure 1.
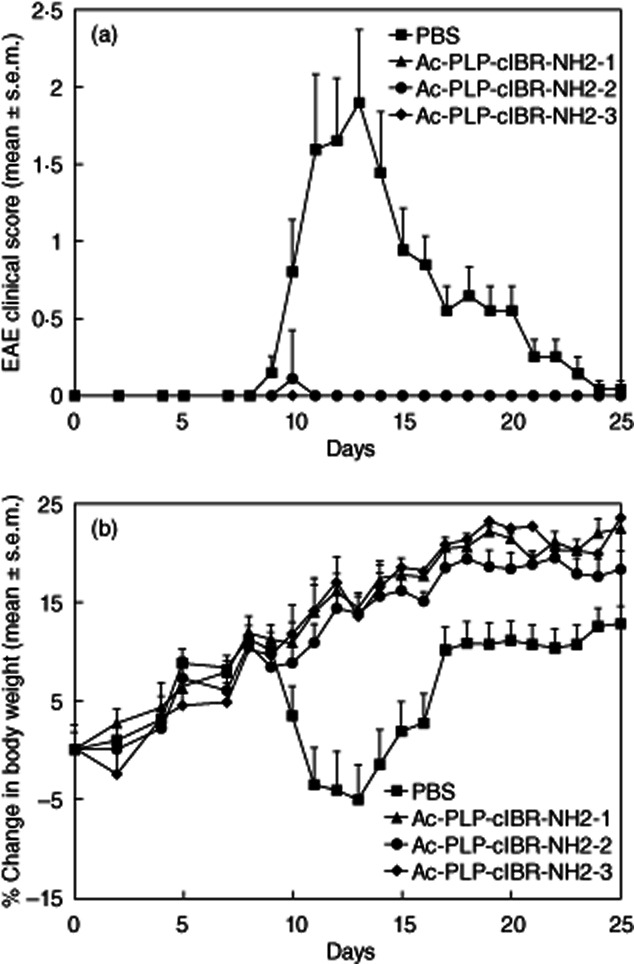
In-vivo efficacies of proteolipid protein–cyclo(1,8)-CPRGGSVC-NH2 (PLP–cIBR) derivatives (Ac-PLP-cIBR-NH2-1, -2 and -3) in suppressing experimental autoimmune encephalomyelitis (EAE) in the mouse model upon treatment with the peptide after immunization with PLP139–151/complete Freund's adjuvant (CFA) on day 0. The immunized mice received intravenous injections of the indicated PLP–cIBR peptide (100 nmol/injection/day) on days 4, 7 and 10. The efficacy of the peptide was determined by (a) clinical disease score of EAE and (b) percentage change in body weight. Results are expressed as the mean ± standard error of the mean (n = 10). EAE scores from all PLP–bifunctional peptide inhibitors (BPI)-treated mice were significantly lower (P < 0·001) than those of phosphate-buffered saline (PBS)-treated mice. Loss of body weight was significantly lower (P < 0·001) in PLP–cIBR-treated mice than in those treated with PBS.
