Abstract
The rapidly warming temperatures in high-latitude and alpine regions have the potential to alter the phenology of Arctic and alpine plants, affecting processes ranging from food webs to ecosystem trace gas fluxes. The International Tundra Experiment (ITEX) was initiated in 1990 to evaluate the effects of expected rapid changes in temperature on tundra plant phenology, growth and community changes using experimental warming. Here, we used the ITEX control data to test the phenological responses to background temperature variation across sites spanning latitudinal and moisture gradients. The dataset overall did not show an advance in phenology; instead, temperature variability during the years sampled and an absence of warming at some sites resulted in mixed responses. Phenological transitions of high Arctic plants clearly occurred at lower heat sum thresholds than those of low Arctic and alpine plants. However, sensitivity to temperature change was similar among plants from the different climate zones. Plants of different communities and growth forms differed for some phenological responses. Heat sums associated with flowering and greening appear to have increased over time. These results point to a complex suite of changes in plant communities and ecosystem function in high latitudes and elevations as the climate warms.
Keywords: growth form, season length, snowmelt, thaw degree days
1. Introduction
As Arctic and alpine regions warm in response to climate change, the growing season for plants is expected to increase from earlier snowmelt in the spring, later snow accumulation in the autumn, or both [1–4]. These climatic zones will also experience higher temperatures during the growing season, although most of the warming for high latitudes and high elevations is projected for the cold season [2,4]. Plant phenological responses to these increases in season length and temperature are uncertain, but understanding changes as they occur is essential for predicting future changes in tundra vegetation processes [3,5]. Remote sensing studies indicate earlier green-up, greater greenness, longer green periods and reduced seasonality in Arctic and alpine regions [6–8]. The species and growth forms behind these changes are uncertain, although the expansion of deciduous shrubs is probably a contributing factor [9]. The warming and greening of the Arctic is heterogeneous across the landscape, and the ecological and environmental factors governing this heterogeneity are poorly understood. This heterogeneity could be caused by ecological factors such as differences in species-specific responses to temperature, or environmental factors such as landscape heterogeneity in early- and late-season snow cover. Ground-based phenological observations of individual plant species and growth forms have the potential to improve our understanding of the mechanisms behind the vegetation response to climate warming.
The International Tundra Experiment (ITEX) was designed in 1990 to test the sensitivity of high-latitude and alpine plant phenology and growth to increased temperature using a passive warming experiment [10]. A synthesis after the first 4 years of warming revealed that climate zones (high Arctic, low Arctic and alpine) and species growth forms (graminoid, forb, evergreen shrub and deciduous shrub) differed in their sensitivity to warming [11]. Earlier greening and flowering occurred for all climate zones and among all growth forms. High Arctic species displayed stronger responses to warming than low Arctic and alpine species. Earlier bud break was stronger for shrubs and forbs during the first 2 years of the experiment. Senescence tended to be later in the growing season for all climate zones and growth forms. Although the first ITEX synthesis improved our understanding of tundra plant phenology, the brief time period of the experiment and covariation among environmental factors associated with climate change limited our ability to project the response of plant phenology to future warming.
Intuitively, the development of plants in cold regions should occur more rapidly with warmer air temperatures, but snow cover can complicate the relationships between air temperature and plant phenology. The beginning of the growing season in high-latitude and alpine sites is often determined by loss of snow cover rather than occurrence of temperatures or temperature sums above a threshold [12,13]. Snowmelt in many sites occurs after temperatures suitable for plant growth have been reached. Although timing of snowmelt is in part determined by air temperature [14,15], a deep snow cover will melt after a shallow snow cover within the same temperature regime. As a result, the link between early-season phenological events and mean monthly or growing season air temperatures may be weak because air temperature does not reflect the temperature experienced by the plants until after snowmelt. While the initiation and termination of anthesis and growth in many Arctic plants is related to day length [12,16], the loss of snow cover frequently takes place after day lengths are long and in the Arctic may be continuous [16]. Thus, the primary drivers for initiation of early-season growth and flowering of most tundra plants are probably the timing of snowmelt and the air temperatures over the period following snowmelt. Late-season phenological responses may be related to temperature or photoperiod [11,12], and declining photoperiod has been shown to affect root growth and leaf senescence of tundra plants [12].
Our approach was to use long-term trends and interannual variability across the ITEX control plots to evaluate change in plant phenology in relation to temperature. Specifically, we address the following hypotheses:
(H1) Flower and leaf bud break are occurring earlier as sites become warmer.
Temperatures at many high-latitude and alpine sites have increased substantially since the initiation of the ITEX project [17], and observations indicate that earlier flowering and leaf bud break are a logical consequence of those increases [18].
(H2) Flower and leaf bud break of high Arctic plants should be more sensitive to interannual temperature variation than those of low Arctic and alpine plants.
Plants from the high Arctic occupy the zone with the lowest temperatures and shortest growing season, and therefore are subject to a greater selective pressure for stronger temperature sensitivity than plants from the low Arctic and alpine zones (figure 1) [18]. Furthermore, temperature increases should equate to a greater proportional increase to growing season temperatures in the high Arctic than the other climate zones. This hypothesis is supported by the results of the first ITEX synthesis [11] that reported stronger evidence for earlier anthesis and leaf bud break in response to experimental warming for high Arctic plants than low Arctic and alpine plants.
(H3) Flower and leaf bud break of plants from dry habitats should be more sensitive to temperature increases than those of plants from moist and wet habitats.
Figure 1.

(a) Conceptual diagrams showing relationships between season length and summer temperature for climatic zones of interest and (b) for community types of different moisture status.
Dry communities are typically associated with windswept ridges with thinner snow cover that become snow free earlier in the year, when temperatures are typically cooler, than low-lying moist or wet communities [19] (figure 1). Furthermore, thin snow cover is a poor insulator for heat and results in lower soil temperatures during the winter than moist or wet communities. Consequently, plants in dry communities should be subject to greater temperature limitation and should have greater temperature sensitivity than moist and wet communities.
(H4) Sensitivity to temperature differs among growth forms.
Deciduous shrubs are often among the earliest species to initiate flowering [20] and might be expected to initiate these stages at lower temperatures than other growth forms. The general increase in deciduous shrubs across the Arctic [21] and increases in graminoids and shrubs to experimental warming [22,23] also suggest a strong differential response among growth forms to changes in temperature.
2. Material and methods
(a). Datasets
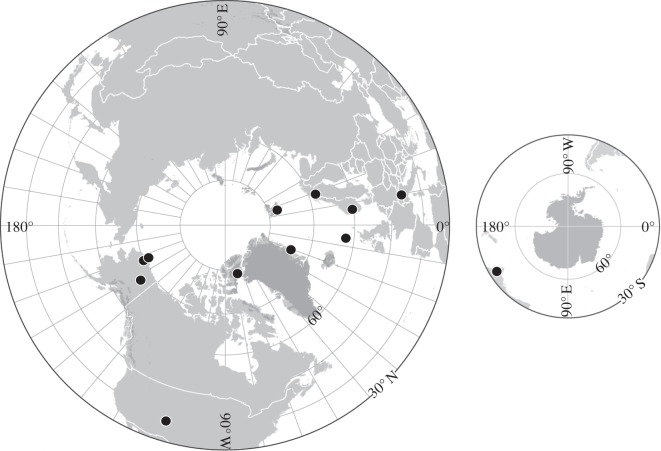
This study examined 12 different sites between 1992 and 2009 (table 1 and figure 2). The study sites span a wide latitudinal range with a 6.9°C difference in mean July temperature (table 1). Data presented here represent the timing (day of year, DOY) of species-level phenological events from the control plots at ITEX sites and related studies that also used protocols outlined in the ITEX Manual [24]. The database included 86 plant species spread across 19 subsites (plant communities within a site). Flowering and leafing stages were compiled from most sites at an observation resolution of one to two times per week. A priority-ranking lumping scheme that accounted for differences in plant morphology was used to consolidate phenological variables, although for a given species at a subsite, the phenological definitions were consistent over time. Rankings were as follows: greening: (i) first leaf visible, (ii) first leaf erect; initial flowering: (i) first flower open, (ii) first anthers exposed, (iii) first flower bud visible; end of flowering: (i) withering of anthers, (ii) first petal drop, (iii) last petal drop; seed maturation: (i) first seed dispersal, (ii) berry ripe; senescence: (i) first colour change, (ii) last colour change, (iii) first leaf drop. Many sites did not measure all five phenological events for each species because of logistical constraints. The most frequently included measurements were first flowering, senescence and greening. A list of sites, subsites, species and events recorded is presented in the electronic supplementary material, table S1.
Table 1.
Characteristics of the study sites included in the analysis. Climate zones: HA, high Arctic; LA, low Arctic; A, alpine. Summer (June–August) air temperatures (Tair) were calculated from 2008 to 1999 or the start of measurements and regressed against year to determine the slope of temperature trend (°C per year); the parentheses enclose F value and d.f., values are in italics when p < 0.10.
| site | subsites (plant communities) | latitude | longitude | elevation ((m).a.s.l.) | climate zone | July Tair (°C) | summer Tair trend (°C yr−1) | years |
|---|---|---|---|---|---|---|---|---|
| Alexandra Fiord, Canada | dry shrub, moist shrub, moist heath | 78°53′ N | 75°45′ W | 10 | HA | 6.7 | 0.06 (2.00,17) | 1992–1998, 2000–2004, 2009 |
| Atqasuk, Alaska | dry heath, wet meadow | 70°27′ N | 157°24′ W | 22 | LA | 9.0 | −0.01 (0.01,15) | 1998–2000, 2007–2008 |
| Barrow, Alaska | dry heath, wet meadow | 71°18′ N | 156°40′ W | 5 | HA | 4.2 | 0.09 (3.67,15) | 1994–2000, 2007–2008 |
| Endalen, Svalbard | moist cassiope (2 sites), dry heath, fellfield, moist snowbed | 78°13′ N | 15°39′ E | 100 | HA | 7.2 | −0.02 (0.04,7) | 2002–2005 |
| Faroe Islands | dry meadow | 62°04′ N | 6°57′ W | 600 | A | 6.9 | −0.05 (0.37,3) | 2002, 2007–2009 |
| Finse, Norway | dry meadow, moist heath, moist snowbed | 60°36′ N | 7°31′ E | 1475 | A | 7.2 | 0.08 (0.52,9) | 1994–1996, 2009 |
| Latnja, Sweden | dry heath, dry meadow, wet tussock, wet sedge | 68°20′ N | 18°30′ E | 1000 | LA | 8.8 | 0.09 (4.25,16) | 1993–1997 |
| Niwot Ridge, Colorado | dry meadow | 40°3′ N, | 105°36′ W | 3528 | A | 10.2 | 0.05 (1.43,17) | 1995–2006, 2008 |
| Bogong High Plains, Australia | dry heath (2 sites) | 36°54′ S | 147°16′ E | 1700 | A | 13.4 | –0.08 (0.06,4) | 2004–2008 |
| Stillberg, Switzerland | moist treeline | 46°46′ N | 9°52′ E | 2180 | A | 10.6 | –0.24 (1.29,7) | 2008–2009 |
| Toolik Field Station, Alaska | moist tussock (2 sites), dry heath | 68°38′ N | 149°38′ W | 720 | LA | 10.3 | –0.04 (0.63,20) | 1996–2001, 2007–2009 |
| Zackenberg, Greenland | dry, moist mixed heath | 74°30′ N | 21°00′ W | 145 | HA | 6.5 | 0.20 (19.5,12) | 1997–2008 |
Figure 2.
Location of the study sites included in this analysis.
The weather dataset was based on data collected at the sites [23] or 0.5° grid data with elevational adjustment using standard lapse rates when weather data were not available [25]. For Finse, Norway, local weather data were available for the more recent, but not the earlier period of the study. In that case, historical data were gap-filled using the relationship between Finse local weather data and data from a nearby weather station. A cubic spline interpolation between recorded average daily temperatures was used to fill smaller gaps (less than one week) in the meteorological record at each site. Data for the Southern Hemisphere site were adjusted six months to synchronize with the larger Northern Hemisphere dataset. Mean monthly air temperatures for the months preceding the growing season and months of the growing season (April–August) were calculated for each study site each year for comparison with plant phenology. Monthly temperatures for each site were tested for the extent of correlation among months using the JMP 10.0.0 statistical package (SAS Institute, Cary, NC, USA). Spring and summer monthly temperatures were significantly correlated within a year (see the electronic supplementary material, table S2), particularly between adjacent months, April and May and June and July (Spearman ρ > 0.9). Consequently, we used mean temperatures of month combinations (spring = April–May and summer = June–August temperatures) as the basis of the temperature analysis. The summer (June–August) temperature trend for each site over the study period is included in table 1.
For those sites where the date of snowmelt was available (most sites), thaw degree days (TDDs) [24] or accumulated heat sums were calculated from the date of snowmelt to the date of each phenological event, where
 |
2.1 |
The timing of species phenological events over the course of a growing season is not independent. Therefore, we tested the degree of correlation of DOY of the three flowering and two leafing events for each species–subsite combination using Spearman rank correlation analysis in JMP. The DOY of the five phenological stages were significantly correlated with each other, especially among the three reproductive phenology parameters and greening (see the electronic supplementary material, table S2). Similar results were also found for TDD (see the electronic supplementary material, table S2). As a result, we present only three of the five phenological stages: flowering, greening and senescence.
To evaluate potential differences in species responses among locations, sites were categorized into climatic zones as in previous syntheses [11,22,23]: high Arctic, low Arctic or alpine. Subsites were categorized as dry, moist or wet, where dry refers to plant communities on well-drained, mineral soils typically located on ridges, moist refers to sites with some soil drainage, and wet refers to plant communities with water tables frequently near or above the surface. To evaluate potential differences between plant growth strategies, species were grouped by growth form (deciduous shrub, evergreen shrub, graminoid or forb).
(b). Response variables
Because site effects such as latitude, elevation and species traits have strong influences on the calendar date that a phenological event occurs, we did not use the DOY associated with a phenological event as a direct measure of phenological response. Instead, we used two types of measures that are largely independent of the site-specific properties (table 2). First, we calculated the TDD from snowmelt until the occurrence of the phenological event for each species-plot combination. This measure reflects the amount of heat accumulated from snowmelt until the phenological event was observed. Second, for each species-subsite combination, we calculated the slopes (β) of the relationships between the timing of the phenological event (represented as DOY or TDD) and the calendar year or site temperature (measured as air temperature of the spring or summer). This approach allowed us to compare responses across species and sites because the slope of the relationship greatly minimizes site and species-specific factors [26]. Negative slopes indicate earlier timing with calendar year or increased temperature and positive slopes indicate delayed timing. In total, we calculated four slopes (β values). To evaluate whether plant phenology was changing over the years of the study, we calculated the slopes of the relationship between DOY of the phenological event and calendar year (βDOY_x_YEAR). Likewise, we calculated the slope of the relationship between TDD of the phenological event and calendar year (βTDD_x_YEAR) to evaluate if the heat sums associated with a phenological event have changed over time. Finally, we calculated the slope of the relationship between DOY of the phenological event and air temperature for both spring (βDOY_x_TAIRSPRING) and summer (βDOY_x_TAIRSUMMER) using the mean daily April–May temperature for spring and June–August temperature for summer. The slope of this relationship (β in days °C−1) has been termed the temperature sensitivity by other authors [26], because it estimates the plant phenological response to changes in temperature. However, these relationships may be influenced by snowmelt patterns; for example, in 2 years with similar air temperatures, phenological events may occur later in years with deeper snow and hence later snowmelt. Spring temperatures used here (April–May) generally correspond to a period when snow cover is often still complete in high Arctic sites. In contrast, loss of snow cover usually begins in mid or late May in low Arctic sites and may be even earlier for alpine sites, depending on elevation and snowfall. Summer temperatures (June–August) correspond to a predominately snow-free period for low Arctic and alpine sites, whereas some snow cover often persists into June for high Arctic sites.
Table 2.
Response variables used for phenological analysis and their derivations.
| response variable | derivation | units | tests |
|---|---|---|---|
| TDD | 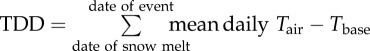 |
°d | accumulation of heat sums |
| βDOY_x_YEAR | slope of date of phenological event versus year of measurement | d yr−1 | temporal trends in timing of phenological events |
| βTDD_x_YEAR | slope of TDD at phenological event versus year of measurement | °d yr−1 | temporal trends in heat sums of phenological events |
| βDOY_x_TAIRSPRING | slope of date of phenological event versus spring air temperature (April–May) | d °C−1 | sensitivity of timing of phenological event to spring air temperature |
| βDOY_x_TAIRSUMMER | slope of date of phenological event versus summer air temperature (June–August) | d °C−1 | sensitivity of timing of phenological event to summer air temperature |
(c). Statistical analysis
To determine whether observed trends in the timing of phenological events were associated with summer temperature trends, we tested the relationship between (βDOY_x_YEAR) with the summer temperature trend (βDOY_x_TAIRSUMMER) for each species at each subsite using linear mixed models, with site, subsite (nested within site) and species as random factors in JMP. Also using JMP, TDD associated with specific phenological events were compared among climate zones, community types and growth forms using linear mixed models, with site, subsite (nested within site) and species as random factors and average TDD for each species–subsite combination as the response variable. Because mean TDD was exponentially distributed, TDD was log-transformed for the analysis.
For analysis of slope (β) values, we used the statistical environment R v. 2.152 [27] to test intercept-only linear mixed models where y = slopes (β values) from a univariate regression for each species, subsite and phenological event combination. That is, the response used was the estimate per species × subsite × phenophase of change in DOY per °C or per year. Random intercepts were included for subsite nested within site, and species (crossed random effect). We ran the same (intercept-only) analyses for different subsets of the data, either including all data, or subsetting by climate zone, community type or growth form. We should note that in some subsets, where not all phenological events were measured at all sites, the comparative differences in the direction and magnitude of the responses of the three phenological events may in part be a result of the absence of data from a site (e.g. a strong response of flowering but a weak response in greening might be because one or more sites did not measure greening). We were unable to test all subsets in a single model because of dataset imbalances. Consequently, results should be considered suggestive because of possible non-independence among factors (e.g. more dry sites in the high Arctic, more deciduous shrubs in the low Arctic, etc.). Significance was assessed by deriving 95% credible intervals for the intercept using Markov Chain Monte Carlo methods to sample from the posterior distribution. If the credible intervals did not span zero the response was considered significantly different from zero. We also re-ran the analysis using a case-resampling bootstrap approach, where we resampled the existing dataset 10 000 times and formed confidence intervals based on the 0.025 and 0.975 quantiles of the resulting distribution of the estimated grand mean slope. Results were similar to those of the previous analysis but exhibited narrower confidence intervals. We present the more conservative results of the first analysis here. In all analyses, we omitted studies (study = species × phenophase × subsite) that lacked at least 3 years of phenology measurements, responses that were not recorded in at least three sites and a handful of studies with small datasets where the model did not converge. Most of these cases applied to alpine sites.
3. Results
(a). Overall responses
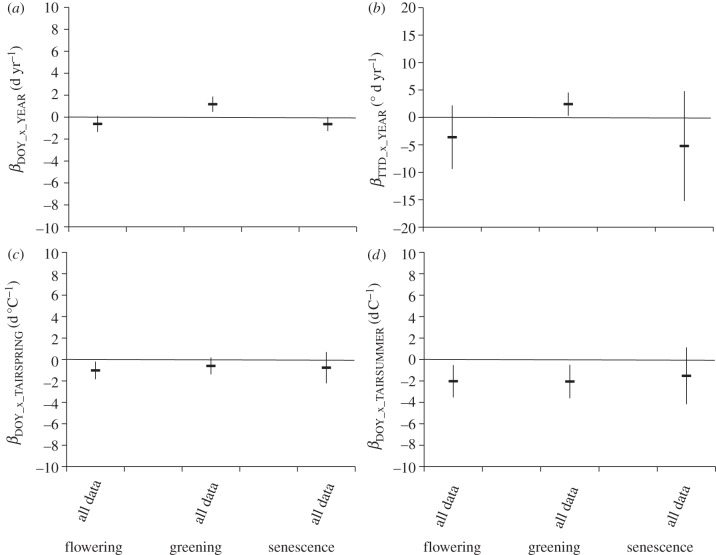
Using all datasets with 3 years or more of data, we tested the significance of the β values (slopes) of the relationships between the timing of the phenological event (represented as DOY or TDD) and the calendar year or site temperature (measured as air temperature of the spring or summer, figure 3). Trends in the timing of events as represented by βDOY_x_YEAR showed significantly later greening (positive slope) but tendencies for earlier flowering and earlier senescence (negative slopes) over the study. Similarly, temporal trends of heat sums (βTDD_x_YEAR) showed significantly increased heat sums over time for greening but a tendency for lower heat sums over time for flowering and senescence. All three phenological events tended towards earlier occurrence in response to higher spring and summer temperatures, with the effect significant for flowering for βDOY_x_TAIRSPRING and flowering and greening for βDOY_x_TAIRSUMMER. The responses to summer temperatures were stronger than those to spring temperatures. Senescence tended to occur earlier in response to warmer spring and summer temperatures, but the slopes were not significantly different from zero.
(H1) Flower and leaf bud break are occurring earlier as sites become warmer.
Figure 3.
Test of significance of slopes (β) of the relationships between the timing of the phenological event (represented as DOY or TDD) and the calendar year or site temperature (measured as air temperature of the spring or summer, table 2). Values represent the mean slope and 95% credible interval (see §2) of the relationships using all data: (a) the relationship between DOY at each phenophase and year (βTDD_x_YEAR); (b) the relationship between TDDs at each phenophase and year (βTDD_x_YEAR); (c) the relationship between DOY at each phenophase and mean spring (April–May) temperature (βDOY_x_TAIRSPRING), and (d) the relationship between DOY at each phenophase and mean summer (June–August) temperature (βDOY_x_TAIRSUMMER).
The mean summer temperature trend of the 12 sites over the study period was positive but not significantly different from zero (mean = 0.012 ± 0.032 s.e. °C yr−1, p = 0.71). Broken down by site, slopes ranged from +0.20 to −0.24 days per year over the time periods when phenology was measured (table 1). The strongest trends were for increasing temperatures at the high Arctic sites, Zackenberg and Barrow, and the low Arctic site, Latjna.
These site differences in temperature trends likely contributed importantly to the overall model results (figure 3a). For example, high Arctic sites were the primary drivers of the significantly later greening and earlier senescence (figure 4), but did not contribute to the overall tendency for earlier flowering.
Figure 4.
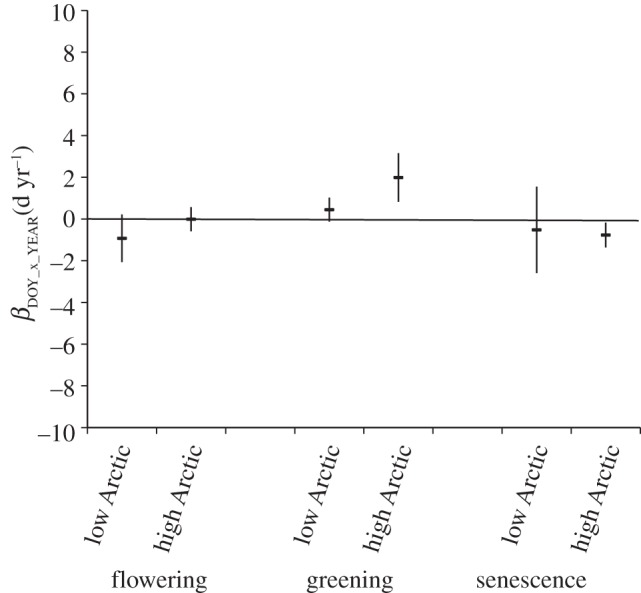
The change in phenology over time; values represent the mean slope and 95% credible interval (see §2) of the relationship between the DOY of each phenophase and study year (βDOY_x_YEAR) for all species at each site × subsite combination partitioned by climate zone (low Arctic, high Arctic) for each phenological event (flowering, greening, senescence). Data were insufficient to test the alpine zone.
We tested whether changes in phenology over time were associated with warming trends by evaluating the relationships between the temporal trend of phenological events (βDOY_x_YEAR) and the summer temperature trend (table 1) for each species × subsite combination. We found strong negative slopes indicating that warmer temperatures were related to earlier flowering (F = 6.72, p < 0.001) and earlier senescence (F = 10.92, p < 0.001; table 3).
(H2) Flower and leaf bud break of high Arctic plants should be more sensitive to temperature increases than those of low Arctic and alpine plants.
Table 3.
Results of mixed models testing the relationship between changing timing of phenology (βDOY_x_YEAR) and changing summer air temperatures (βDOY_x_TAIRSUMMER) by phenological event for each species × subsite combination. As a result of data limitations, the model did not converge for green.
| stage | R2adj | slope | d.f. | t | p-value |
|---|---|---|---|---|---|
| flower | 0.73 | −0.306 | 1 | 6.72 | <0.0001 |
| green | — | — | — | — | — |
| senesce | 0.90 | −0.134 | 1 | 10.92 | <0.0001 |
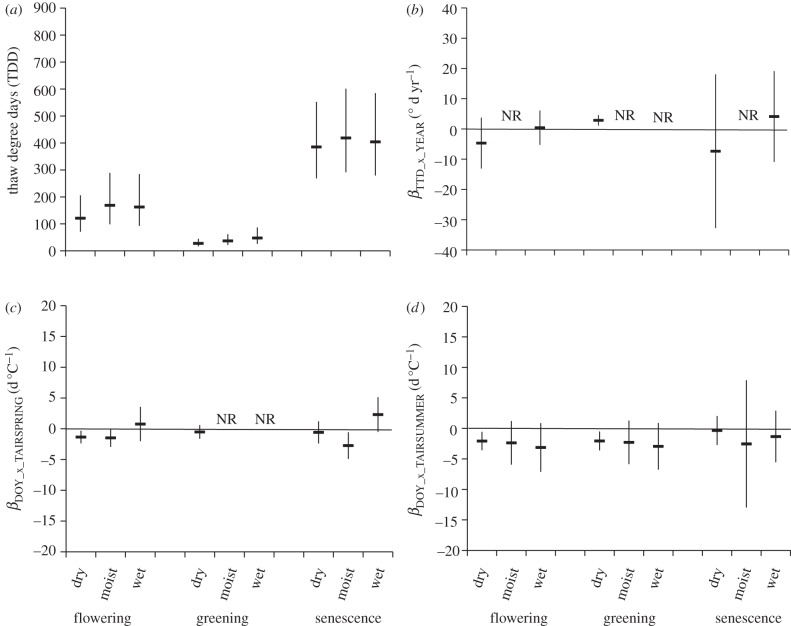
The TDD for flowering (F = 10.3, p = 0.014) and senescence (F = 32.4, p = 0.019) differed strongly among climate zones (figure 5); flowering, greening and senescence of high Arctic plants generally occurred at lower TDD than those of low Arctic and alpine plants in that order.
Figure 5.
Differences in phenological response among climate zones (alpine, low Arctic and high Arctic). (a) The least-squares mean TDD ± s.e. attained at each phenological event (flowering, greening and senescence) by climate zone. The mean slope and 95% credible intervals of the relationship between: (b) TDDs at each phenophase and year (βTDD_x_YEAR); (c) DOY at each phenophase and mean spring (April–May) temperature (βDOY_x_TAIRSPRING); and (d) DOY at each phenophase and mean summer (June–August) temperature (βDOY_x_TAIRSUMMER) for each phenological event partitioned by climate zone. NR, not reported–insufficient data.
Temporal trends in TDD (βTDD_x_YEAR) for flowering and greening events were generally greater than zero for both low and high Arctic plants (figure 5), indicating that thermal thresholds for flowering and greening have increased over time at these sites. Negative temporal trends in TDD (βTDD_x_YEAR) for alpine plants failed to converge on a solution, but had a strong effect on the overall model for flowering (figure 3a). Temporal trends in TDD for senescence were generally less than zero, but were not statistically significant.
The slopes of the relationships between DOY and mean spring and summer air temperatures (βDOY_x_TAIRSPRING and βDOY_x_TAIRSUMMER) tended, as expected, towards negative values (earlier occurrence of phenological event with higher temperature) for high Arctic flowering and low Arctic greening (figure 5). Responses to summer temperatures were stronger and uniformly negative. The response of high and low Arctic plants to summer temperatures were similar for both flowering and greening. The tendency across sites was for earlier senescence with warmer spring and summer temperatures.
(H3) Flower and leaf bud burst of plants from dry habitats should be more sensitive to temperature increases than those of plants from moist and wet habitats.
The TDD differed significantly among community types for flowering (F = 11.2, p = 0.013) and nearly so for greening (F = 4.70, p = 0.0517). TDDs were lowest for plants at dry sites for both flowering and greening (figure 6). TDD for senescence was similar across all community types. Temporal trends of TDD (βTDD_x_YEAR) showed a significant trend towards greater TDD in recent years at the dry sites; the model could not converge on values for moist sites (figure 6). The relationship between flowering and spring temperatures (βDOY_x_TAIRSPRING) was significantly less than zero for dry and moist sites (figure 6), indicating earlier flowering with warmer temperatures. Only dry sites had sufficient data in the model for greening and they were not different from zero. Moist sites showed significantly earlier senescence with warmer spring temperatures. For summer temperatures, all community types tended to show earlier flowering and greening (negative βDOY_x_TAIRSUMMER values) with warmer summer temperatures, and these were statistically significant at the dry sites. Values of βDOY_x_TAIRSUMMER for senescence were not significantly different from zero.
(H4) Sensitivity to temperature differs among growth forms.
Figure 6.
Differences in phenological response among community types (dry, moist and wet). (a) The least-squares mean TDD ± s.e attained at each phenological event (flowering, greening and senescence) by community types. The mean slope and 95% credible intervals of the relationship between: (b) TDDs at each phenophase and year (βTDD_x_YEAR); (c) DOY at each phenophase and mean spring (April–May) temperature (βDOY_x_TAIRSPRING); and (d) DOY at each phenophase and mean summer (June–August) temperature (βDOY_x_TAIRSUMMER) for each phenological event partitioned by community types. NR, not reported–insufficient data.
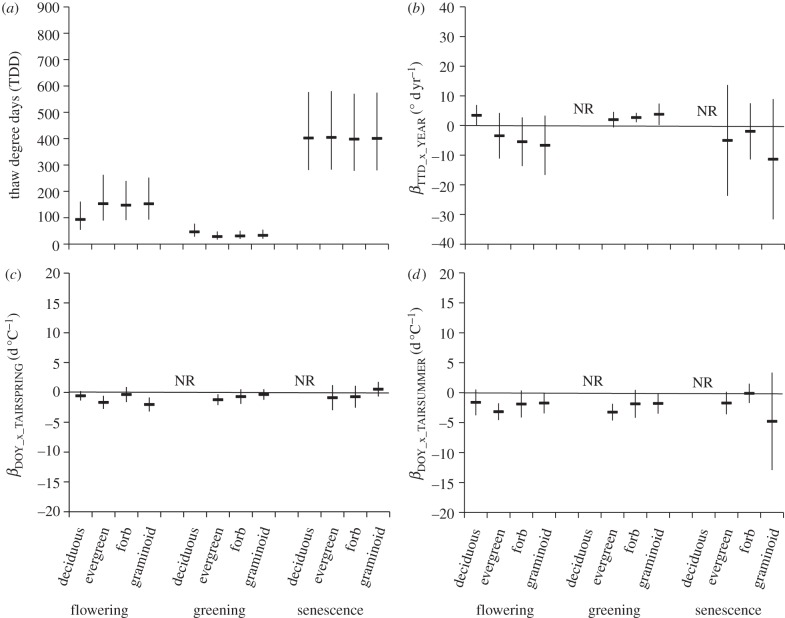
Deciduous shrubs flowered at lower TDD than other growth forms (figure 7). In contrast, greening of deciduous shrubs occurred at the highest TDD. TDD at senescence was similar among all growth forms. Temporal trends of TDD were mixed with deciduous shrubs showing greater TDD in recent years, whereas other growth forms trended in the opposite direction (figure 7). Deciduous shrubs did not converge in the model for the other phenological events. Evergreens, forbs and graminoids trended towards greater TDD in recent years for greening. Mean βTDD_x_YEAR for senescence were all negative but not significantly different from zero.
Figure 7.
Differences in phenological response among growth forms (deciduous shrub, evergreen shrub, forb and graminoid). (a) The least-squares mean TDD ± s.e. attained at each phenological event (flowering, greening and senescence) by growth form. The mean slope and 95% credible intervals of the relationship between: (b) TDDs at each phenophase and year (βTDD_x_YEAR); (c) DOY at each phenophase and mean spring (April–May) temperature (βDOY_x_TAIRSPRING); and (d) DOY at each phenophase and mean summer (June–August) temperature (βDOY_x_TAIRSUMMER) for each phenological event partitioned by growth form. NR, not reported–insufficient data.
The relationships between spring temperatures and flowering and greening (βDOY_x_TAIRSPRING) did not differ among growth forms and were significantly negative (meaning warmer temperatures result in earlier phenology) for flowering of evergreens and graminoids and greening of evergreens (figure 7). A similar relationship existed between summer temperatures and flowering or greening (βDOY_x_TAIRSUMMER). Senescence tended to occur earlier with warmer summer temperatures but this trend was not significant for any growth form.
4. Discussion
(a). Timing of phenological events
Temperatures of some of the long-term ITEX sites, especially those in the high Arctic, have increased strongly in the past two decades (table 1), as have temperatures overall for high latitudes and high elevations [4,5,17]. As phenology is one of the most responsive plant traits to climate warming [3,24], we hypothesized that plant phenology would show evidence of shifts towards earlier dates across all sites. However, we found that phenological trends were site dependent and probably a result of the different temperature trends among the sites over the years included in the study. For leaf bud burst, the overall temporal trend was towards later greening across all sites (figure 3a), but when the magnitude of warming at each site was taken into account bud break phenology was responsive to temperature and advanced with warming (figure 3c,d and table 3).
Unexpectedly, temperature thresholds for greening and flowering of both high and low Arctic plants have tended to increase over time (figure 5b). If phenological events are responding primarily to heat sums, a test of TDD across years should show relatively constant TDD for phenological event versus year. Our findings imply that as temperatures have warmed, critical heat sum thresholds for greening are rising. A number of factors may be contributing to this result. As spring temperatures increase, resulting in earlier snowmelt, the probability of post-snowmelt freezing events that adversely affect plant development may increase [4]. While air and soil temperatures are generally coupled, warm spring temperatures immediately post-snowmelt may increase TDD rapidly while soils remain cold and plant growth is still limited [28,29]. Plants may not be meeting their chilling requirements as a result of warmer winter temperatures [20]. Alternatively, plants may be acclimating to increased temperatures, with a stabilizing effect on timing of phenological stage. While heat sums are the standard measurement associated with phenological stage in cold regions, limitations with the utility of heat sums for prediction of phenology have been previously recognized [20,30,31]. We also cannot entirely discount the possibility that as the rate of heat sum accumulation increased and phenological events occurred earlier with higher temperatures, earlier phenological events were undetected because the frequency of phenological measurements remained constant over the study.
(b). Comparison among climatic zones
We found clear evidence that high Arctic species flower, green and senesce at lower TDD than low Arctic and alpine plants. This result is consistent with the decrease in TDD needed for flowering with increasing latitude [11,32]. However, the relationships between phenology and average summer temperatures were very similar for high and low Arctic plants. These results suggest that high Arctic plants operate at lower temperatures, but the response to temperature increases is similar to those of low Arctic plants.
Different from the first ITEX synthesis that suggested delayed senescence with experimental warming [11], results from this study indicate earlier senescence with warmer temperatures. Earlier senescence of high Arctic plants in particular was associated with warmer spring temperatures.
(c). Comparison among community types
We hypothesized that plants in dry sites would be more sensitive to temperature because dry sites generally have lighter snow cover, warmer soils and emerge from snow earlier than moist and wet sites [19,33]. The data supported our hypothesis with plants on dry sites flowering and greening at lower heat sums than plants of moist and wet sites. Dry sites were also the only sites to show significant relationships between increased spring and summer temperatures on earlier flowering or greening (figure 6c,d). Wet sites, which typically have the deepest snow cover and are the last to warm, showed no response to spring temperatures (figure 6c).
(d). Comparison among growth forms
We had hypothesized difference in the phenological response to temperature among growth forms based on the widespread increase in shrub growth [21], findings of the first ITEX synthesis [11], and the use of plant functional types for prediction of transient responses to change [34]. Flowering of deciduous shrubs occurred at the lowest accumulated heat sums of any growth form, but greening of deciduous shrubs occurred at higher TDD than most other growth forms. Previous reports have suggested that graminoids often green up earliest, and forbs, with their buried buds, are often the last to leaf out [12]. Evergreens green up quickly upon snow melt, but actual bud break is typically the latest of all growth forms [12]. Low TDD for evergreens noted here may be a result of the use of greening to refer to loss of protective pigmentation rather than bud break [35]. Evergreens and graminoids consistently showed earlier greening and flowering in response to warmer temperatures, but all growth forms senesced at similar TDD values.
(e). Overall temperature response
A recent analysis of changes in phenology [26] reported that plants from a wide range of habitats and climate regimes have a temperature sensitivity of approximately 4 and 7 days per °C increase in mean annual air temperature for flowering and leafing, respectively. When the temperature sensitivity of individual species in the present study was estimated by using the slope of the relationship between DOY and summer air temperature, the mean temperature response was a decrease of 2.4, 1.6 and 1.9 days per °C in mean summer temperature for flowering, greening and senescence, respectively. Because these values are influenced by timing of snowmelt, these results may be in part a consequence of variation in timing of snowmelt among years. An additional complication is that in some alpine areas, greening and flowering of plants has been decoupled from timing of snowmelt as a result of early snowmelt caused by dust deposition from adjacent lowlands [36]. Black carbon from combustion and forest fires transported to high latitudes and elevations may have similar influences [37].
(f). Season length
While most of the analyses of alpine sites in the present study did not converge on a solution or were filtered by the analysis criteria, the overall pattern for alpine sites was earlier flowering and leafing with warming and a longer growing season, as was found in the first ITEX synthesis [11]. In contrast to alpine sites but somewhat similar to our findings for greening in the high and low Arctic, satellite observations of the alpine Tibetan Plateau have suggested that as temperatures have risen, spring phenology of the dominant vegetation has been delayed, leading to a shorter growing season [38]. The authors suggest that warmer winter temperatures led to later fulfillment of plant chilling requirements, which delayed spring green-up. A study of deciduous shrubs from a low Arctic site, however, found evidence that chilling requirements were met very early in the winter and were unlikely to limit to spring bud break [20]. A recent reanalysis of the long-term satellite datasets identified quality problems, and concluded that phenology has indeed advanced across the Tibetan Plateau over the past 30 years [39]. On the other hand, experiments in central Tibet have found that reproductive phenology of the dominant vegetation was delayed in response to warming, a finding attributed to soil drying as a result of the warming [40]. Furthermore, indigenous observations of climate and ecological change in Tibet have also reported delayed and shortened summer seasons (J. Klein et al., unpublished data).
Leaf senescence showed somewhat conflicting responses in the present study, with some subsets showing advanced senescence with higher temperature and others delayed senescence. In the first ITEX synthesis, the response of senescence to warming was a relatively weak delay [11]. The weak response was attributed to controlling effects of photoperiod on senescence, for which evidence is strong [12]. However, for some deciduous shrubs and forbs, senescence may be determined by leaf age; plants that produce a single early growth flush senesce when the maximum leaf age is attained. This response has been demonstrated experimentally for deciduous shrubs [41] and a forb species [42] in which earlier bud break led to earlier senescence. In contrast, species that spread leaf production over the growing season (some graminoids, for example) might be able to take advantage of the longer warm period [12]. A study of alpine plants in response to snow cover and temperature variation [43] reported that warmer temperatures following snowmelt consistently shortened the growing season. A likely mechanism for shortened growing seasons may be early senescence in response to warming-induced soil drying.
(g). Significance
With future warming and drying combined with permafrost degradation in tundra regions, the location and proportion of dry, moist and wet habitats will shift substantially [44,45]. An understanding that the temperature responses of the phenological stages for plants of various community types and growth forms may differ should be extremely useful for predicting the composition and function of future high-latitude and high-elevation plant communities as they change with climate warming. Among the important responses was a reduction in the effective growing season with warming as a result of early senescence. Whether the basis for this response is related to warming-induced drying remains to be seen.
The finding that species from the high Arctic had the lowest heat sum thresholds was expected given the low-temperature environments in which these plants function and confirms the experimental comparison in the first ITEX synthesis [11]. This result suggests the presence of physiological differences among plants from the three climate zones (e.g. respiration and photosynthetic tolerance to temperature) that influence important ecosystem processes. However, the data do not support different temperature sensitivity among the different climate zones. In fact, the relationship between phenology and summer temperature was essentially the same for high and low Arctic sites. These findings suggest that the recent strong greening in the high Arctic coastal regions detected via remote sensing [46] is a response to greater warming rather than greater sensitivity of the plants found there.
Acknowledgements
We gratefully acknowledge the tremendous efforts by the army of technicians who conducted phenology measurements at the study sites. Two anonymous reviewers provided very helpful comments to improve the manuscript.
Data accessibility
Logistical support of the NCEAS group is greatly appreciated. Data are archived at ACADIS (www.aoncadis.org). Norway weather data were obtained from the Eklima system, and Toolik weather data from the Arctic LTER database.
Funding statement
This work is based in part on support by the United States National Science Foundation awards OPP-0632277 and OPP-0856710 to S.F.O. and W.A. Gould, NCEAS synthesis workshop funds to S.F.O. and T.G.T. and a grant from the Canadian International Polar Year program to G.H.R.H. Additional support came from the site-specific funding sources.
References
- 1.Maxwell B. 1992. Arctic climate: potential for change under global warming. In Arctic ecosystems in a changing climate: an ecophysiological perspective (eds Chapin FS, III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J.), pp. 11–34 San Diego, CA: Academic Press [Google Scholar]
- 2.Overpeck J, et al. 1997. Arctic environmental change of the last four centuries. Science 278, 1251–1256 (doi:10.1126/science.278.5341.1251) [Google Scholar]
- 3.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. 2007. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365 (doi:10.1016/j.tree.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 4.IPCC (Intergovernmental Panel on Climate Change) 2007. Climate Change 2007: impacts, adaptation and vulnerability. Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.ACIA 2005. Impacts of a warming Arctic: Arctic climate impact assessment. Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Myneni RB, Keeling D, Tucker J, Asrar G, Nemani RR. 1997. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386, 698–702 (doi:10.1038/386698a0) [Google Scholar]
- 7.Jia GS, Epstein HE, Walker DA. 2003. Greening of Arctic Alaska, 1981–2001. Geophys. Res. Lett. 30, 2067 (doi:10.1029/2003GL018268) [Google Scholar]
- 8.Xu L, et al. In press. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Change. (doi:10.1038/nclimate1836) [Google Scholar]
- 9.Forbes BC, Fauria M, Zetterberg P. 2009. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob. Change Biol. 16, 1542–1554 (doi:10.1111/j.1365-2486.2009.02047.x) [Google Scholar]
- 10.Webber PJ, Walker MD. 1991. The International Tundra Experiment (ITEX): resolution. Arct. Alpine Res. 23, 124 (doi:10.2307/1551451) [Google Scholar]
- 11.Arft AM, et al. 1999. Responses of tundra plants to experimental warming: meta-analysis of the International Tundra Experiment. Ecol. Monogr. 69, 491–511 [Google Scholar]
- 12.Shaver GR, Kummerow J. 1992. Phenology, resource allocation, and growth of Arctic vascular plants. In Arctic ecosystems in a changing climate: an ecophysiological perspective (eds Chapin FS, III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J.), pp. 193–211 San Diego, CA: Academic Press [Google Scholar]
- 13.Olsson PQ, Sturm M, Racine CH, Romanovsky V, Liston GE. 2003. Five stages of the Alaskan Arctic cold season with ecosystem implications. Arct. Antarct. Alpine Res. 35, 74–81 (doi:10.1657/1523-0430(2003)035[0074:FSOTAA]2.0.CO;2) [Google Scholar]
- 14.Kane DL, Hinzman LD, Woo M, Everett KR. 1992. Arctic hydrology and climate change. In Arctic ecosystems in a changing climate: an ecophysiological perspective (eds Chapin FS, III, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J.), pp. 35–57 San Diego, CA: Academic Press [Google Scholar]
- 15.Lambert AM, Miller-Rushing AJ, Inouye DW. 2010. Changes in snowmelt date and summer precipitation affect the flowering phenology of Erythronium grandiflorum (glacier lily; Liliaceae). Am. J. Bot. 97, 1431–1437 (doi:10.3732/ajb.1000095) [DOI] [PubMed] [Google Scholar]
- 16.Chapin FS, III, Shaver GR. 1985. Arctic. In Physiological ecology of North American plant communities (eds Chabot BF, Mooney HA.), pp. 16–40 New York, NY: Chapman and Hall [Google Scholar]
- 17.Wendler G, Shulski M, Moore B. 2010. Changes in the climate of the Alaskan north slope and the ice concentration of the adjacent Beaufort Sea. Theoret. Appl. Climatol. 99, 67–74 (doi:10.1007/s00704-009-0127-8) [Google Scholar]
- 18.Billings WD. 1987. Constraints to plant growth, reproduction, and establishment in Arctic environments. Arct. Alpine Res. 19, 357–365 (doi:10.2307/1551400) [Google Scholar]
- 19.Walker DA, Halfpenny JC, Walker MD, Wessman CA. 1993. Long-term studies of snow-vegetation interactions. BioScience 43, 287–301 (doi:10.2307/1312061) [Google Scholar]
- 20.Pop EW, Oberbauer SF, Starr G. 2000. Predicting bud break in two Arctic deciduous shrubs: Betula nana and Salix pulchra . Oecologia 124, 176–184 (doi:10.1007/s004420050005) [DOI] [PubMed] [Google Scholar]
- 21.Sturm M, Racine C, Tape K. 2001. Increasing shrub abundance in the Arctic. Nature 411, 546–547 (doi:10.1038/35079180) [DOI] [PubMed] [Google Scholar]
- 22.Walker MD, et al. 2006. Plant community responses to experimental warming across the tundra biome. Proc. Natl Acad. Sci. USA 103, 342–346 (doi:10.1073/pnas.0503198103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmendorf SC, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175 (doi:10.1111/j.1461-0248.2011.01716.x) [DOI] [PubMed] [Google Scholar]
- 24.Molau U, Mølgaard PE. 1996. ITEX manual. Copenhagen, Denmark: Danish Polar Centre [Google Scholar]
- 25.Jones P, Harris I. 2008. CRU Time Series (TS) high resolution gridded datasets, [Internet]. NCAS British Atmospheric Data Centre, University of East Anglia Climate Research Unit (CRU). See http://badc.nerc.ac.uk/data/cru/.
- 26.Wolkovich EM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497 [DOI] [PubMed] [Google Scholar]
- 27.R-Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 28.Kummerow J, McMaster GS, Krause DA. 1980. Temperature effect on growth and nutrient contents in Eriophorum vaginatum under controlled environmental conditions. Arct. Alpine Res. 12, 335–342 (doi:10.2307/1550719) [Google Scholar]
- 29.Starr G, Neuman DS, Oberbauer SF. 2004. Ecophysiological analysis of two Arctic sedges under reduced root temperatures. Physiol. Plantarum 120, 458–464 (doi:10.1111/j.0031-9317.2004.00260.x) [DOI] [PubMed] [Google Scholar]
- 30.Hänninen H. 1995. Effects of climatic change on trees from cool and temperate regions: an ecophysiological approach to modeling of bud burst phenology. Can. J. Bot. 73, 183–199 (doi:10.1139/b95-022) [Google Scholar]
- 31.Wipf S, Rixen C, Mulder CP. 2006. Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Glob. Change Biol. 12, 1496–1506 (doi:10.1111/j.1365-2486.2006.01185.x) [Google Scholar]
- 32.Shaver GR, Fetcher N, Chapin FS. 1986. Growth and flowering in Eriophorum vaginatum: annual and latitudinal variation. Ecology 67, 1524–1535 (doi:10.2307/1939083) [Google Scholar]
- 33.Walker DA, Binnian E, Evans BM, Lederer ND, Nordstrand E, Webber PJ. 1989. Terrain, vegetation and landscape evolution of the R4D research site, Brooks Range foothills, Alaska. Ecography 12, 238–261 (doi:10.1111/j.1600-0587.1989.tb00844.x) [Google Scholar]
- 34.Chapin FS, Bret-Harte MS, Hobbie SE, Zhong HL. 1996. Plant functional types as predictors of transient responses of Arctic vegetation to global change. J. Veg. Sci. 7, 347–358 (doi:10.2307/3236278) [Google Scholar]
- 35.Oberbauer SF, Starr G. 2002. The role of anthocyanins for photosynthesis of Alaskan Arctic evergreens during snow melt. Adv. Bot. Res. 37, 129–145 (doi:10.1016/S0065-2296(02)37047-2) [Google Scholar]
- 36.Steltzer H, Landry C, Painter TH, Anderson J, Ayres E. 2009. Biological consequences of earlier snowmelt from desert dust deposition in alpine landscapes. Proc. Natl Acad. Sci. USA 106, 11 629–11 634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flanner MG, Zender CS, Randerson JT, Rasch PJ. 2007. Present-day climate forcing and response from black carbon in snow. J. Geophys. Res. 112, D11202 (doi:10.1029/2006JD008003) [Google Scholar]
- 38.Yu HY, Luedeling E, Xu JC. 2010. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl Acad. Sci. USA 107, 22151–22156 (doi:10.1073/pnas.1012490107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang G, Zhang Y, Dong J, Xiao X. 2013. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. Proc. Natl Acad. Sci. USA 110, 4309–4311 (doi:10.1073/pnas.1210423110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorji T, Totland Ø, Moe SR, Hopping KA, Pan J, Klein JA. 2013. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob. Change Biol. 19, 459–472 (doi:10.1111/gcb.12059) [DOI] [PubMed] [Google Scholar]
- 41.Oberbauer SF, Starr G, Pop EW. 1998. Effects of extended growing season and soil warming on carbon dioxide and methane exchange of tussock tundra in Alaska. J. Geophys. Res. 103, 29 075–29 082 (doi:10.1029/98JD00522) [Google Scholar]
- 42.Starr G, Oberbauer SF, Pop EW. 2000. Effects of extended growing season and soil warming on phenology and physiology of Polygonum bistorta. Glob. Change Biol. 6, 357–369 (doi:10.1046/j.1365-2486.2000.00316.x) [Google Scholar]
- 43.Jonas T, Rixen C, Sturm M, Stoeckli V. 2008. How alpine plant growth is linked to snow cover and climate variability . J. Geophys. Res. 113, G03013 (doi:10.1029/2007JG000680) [Google Scholar]
- 44.Hinzman LD, et al. 2005. Evidence and implications of recent climate change in northern Alaska and other Arctic regions. Clim. Change 72, 251–298 (doi:10.1007/s10584-005-5352-2) [Google Scholar]
- 45.Smith LC, Sheng Y, MacDonald GM, Hinzman LD. 2005. Disappearing Arctic lakes. Science 308, 1429 (doi:10.1126/science.1108142) [DOI] [PubMed] [Google Scholar]
- 46.Bhatt US, et al. 2010. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interact. 14, 1–20 (doi:10.1175/2010EI315.1) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Logistical support of the NCEAS group is greatly appreciated. Data are archived at ACADIS (www.aoncadis.org). Norway weather data were obtained from the Eklima system, and Toolik weather data from the Arctic LTER database.