Abstract
Previous studies have shown that rat epididymis-specific gene HongrES1 plays important roles in sperm capacitation and fertility. In this study, we cloned the mouse homologue gene by sequence alignment and RT-PCR methods and designated it as mHong1. The mHong1 gene is located on chromosome 12p14, spanning five exons. The cDNA sequence consists of 1257 nucleotides and encodes a 419 amino-acid protein with a predicted N-terminal signal peptide of 20 amino acids. The mHong1 mRNA shows similarity with HongrES1 in the expression patterns: (i) specific expression in epididymal tissue, especially in the cauda region; and (ii) androgen-dependence but testicular fluid factor independence. Its protein product shows 71% similarity with HongrES1 and contains a classical serpin domain as does HongrES1. A polyclonal antibody against mHong1 with high specificity and sensitivity was raised. Like HongrES1, the mHong1 protein shows a checker-board expression pattern in the epididymal epithelium and is secreted into the epididymal lumen. The mHong1 protein shows higher glycosylation than HongrES1. Although both of them are deposited onto the sperm head surface, mHong1 is localized to the equatorial segment, which is different from that of HongrES1. The mHong1 protein can be removed from the sperm membrane by high ionic strength and therefore can be classed as an extrinsic membrane protein. Collectively, we conclude that mHong1 is the homologue of HongrES1 and the present work paves the way for establishing animal models to elucidate the precise functions of HongrES1 and mHong1.
Keywords: androgen, epididymal secretory protein, epididymis, glycosylation, sperm maturation
Introduction
The epididymis contains a long coiled tubule responsible for sperm transport, protection, storage and maturation.1 It has been shown that some epididymis-specific secretory proteins associate with spermatozoa and play important roles in sperm maturation.2, 3, 4
HongrES1, a new member of the serpin family, was cloned from the rat epididymis by our laboratory.5 It is specifically expressed in the cauda epididymidis and secreted to the lumen and deposited on the sperm head surface. Knockdown of HongrES1 by RNAi in vivo leads to accelerated capacitation but does not affect sperm motility and the acrosome reaction. The about 50% knockdown of HongrES1 protein expression results in impaired fertility, i.e., a considerable decrease in litter size and blastocyst number and the appearance of dead or small-sized offspring and regressing implanted embryos. These results indicate that HongrES1 is a novel and key regulator of sperm capacitation and male fertility.6
In HongrES1 gene knockdown male rats, there are mainly three types of spermatozoa, i.e., those with heads fully, partially and not covered by HongrES1 protein. These HongrES1 binding differences may account for the above-mentioned phenotypes. To clarify the function of HongrES1, it is better to obtain HongrES1 null spermatozoa, but it was impossible to isolate them from the HongrES1 knockdown rats. Therefore, production of HongrES1 knockout mice is the optimal method for which the cloning, identification and characterisation of mouse homologue of HongrES1 is needed.
Recently, the HongrES1-like protein has also been identified in the guinea pig. It was localized to the cauda epididymidis and deposited on the anterior sperm acrosome region. Removal of this protein from the guinea pig sperm surface is associated with capacitation and hyperactivation.7
In this article, we describe the cloning of the HongrES1 homologue, named mHong1, from the mouse epididymis and the characterisation of this gene at both the mRNA and protein levels.
Materials and methods
Animals
Healthy adult male C57BL/6 mice (body weight: 20–25 g) and adult male New Zealand white rabbits (body weight: 2.5 kg) were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Experiments were conducted by following a protocol approved by the Institute Animal Care Committee. The protocol conforms to internationally-accepted guidelines for the humane care and use of laboratory animals.
DNA and protein sequence analysis
The rat HongrES1 sequence (GenBank accession number NM_181630) was used as the sequence to interrogate the mouse genome in the National Center for Biotechnology Information. Two fragments of the genome (224 bp and 657 bp), producing significant alignments, were retrieved. Two pairs of primers (forward primer FP1: 5′-AAGGAGAAGGGTTCCCTTGGTTGC-3′ reverse primer RP1: 5′-CTGAAACCTGTCTGCCAGTGGCT-3′ forward primer FP2: 5′-CCTCATCCCTGTGTACTTCGGGT-3′ reverse primer RP2: 5′-AACTCTGCTACAACTCAGGTAGTG-3′) were synthesized on the basis of the sequences of the two fragments.
The cDNA fragments of mHong1 were amplified by RT-PCR. Total RNA isolated from the mouse epididymis was reverse-transcribed by SuperScript reverse transcriptase (Gibco/BRL, Grand Island, NY, USA) as per the manufacturer's recommendations. The cDNA fragments of 224 bp, 653 bp and 1524 bp were amplified by PCR with primers FP1/RP1, FP2/RP2 and FP1/RP2, respectively, with Ex-Taq (Takara, Dalian, China). The full-length cDNA sequence was obtained by 5′-rapid amplification of cDNA ends (RACE) and 3′-RACE (protocol of the FirstChoice RLM-RACE kit; Ambion, Austin, TX, USA).
RNA extraction and Northern blot analysis
Total RNA was extracted from mouse tissues with Trizol (Invitrogen, Grand Island, NY, USA) by following the manufacturer's instructions. Northern blot analysis was performed as described previously.8 Fifteen micrograms of total RNA from each sample were loaded in each lane. Probes were 32P-labelled mHong1 cDNA fragments (1–224 bp and 872–1524 bp, used only for Figure 1; 225–871 bp, used in all Northern blot experiments). A 18s r-RNA hybridisation signal was used as a loading control. Radio-autographs with pronounced differences in expression were analysed by densitometry.
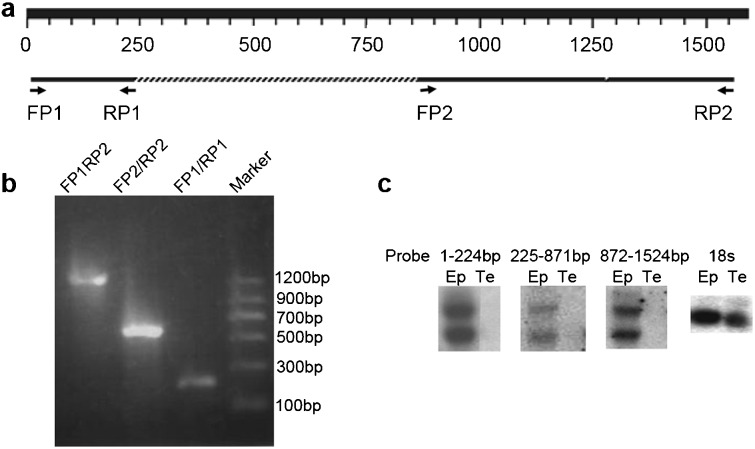
Figure 1.
Cloning of the full-length mHong1. (a) The rat HongrES1 cDNA sequence was used to interrogate the mouse genome. Two fragments (224 bp and 653 bp) with significant similarity were obtained. On the basis of the sequences of these two fragments, two pairs of primers (FP1/RP1, FP2/RP2) were synthesized. (b) Amplification of cDNA fragments of mHong1 by RT-PCR. The cDNA fragments of 224 bp, 653 bp and 1524 bp were amplified with primers FP1/RP1, FP2/RP2 and FP1/RP2, respectively. (c) Northern blot analysis of mHong1 mRNA in the epididymis and testis probed with different cDNA regions (1–224 bp, 225–871 bp and 872–1524 bp). 18s rRNA was used as loading control. Ep, epididymis; Te, testis.
Castration and androgen replacement
Adult (8-week-old) male C57 mice were castrated bilaterally under sodium pentobarbital anesthesia. The mice were divided into nine groups (six mice per group), each killed at different days after castration (0, 1, 3, 5 and 7 days) and 1, 3, 5 and 7 days after a single injection of testosterone propionate (5 mg kg−1 body weight) given to the 7-day castrated mice. Epididymal samples for each group were pooled for RNA extraction. Pooled serum samples from every group were sent to Shanghai Zhongshan Hospital for measurement of testosterone content by radioimmunoassay.9
Efferent duct ligation
Adult (8-week-old) male C57 mice were anaesthetized with sodium pentobarbital and the efferent ducts of the left testis of each animal were ligated with silk thread (braided silk sutures, size 5/0) at their junctions with the extratesticular rete testis, avoiding damage to the regional blood vessels. As controls, the right testes were manipulated similarly to the left testes, but the efferent ducts were not ligated. The mice (n=5) were killed 2 weeks after surgery. Ligated and control epididymides were dissected out and processed for Northern blot analyses.
Anti-mHong1 polyclonal antisera
The cDNA fragments coding for the C-terminal peptide (230–418 amino acids) were amplified by PCR by using the following primers: the forward primer was 5′-GCTAGCACCAATATCCTGGTA-3′, and the reverse primer was 5′- AAGCTTTCAGGGACGATGTAC-3′. The fragment with a NheI site at its 5′ end and a HindIII site at its 3′ end was inserted into the pET28 (a) vector (Novagen, Darmstadt, Germany). The expression vector was constructed according to the standard protocol in the pET expression manual (pET System Manual, tenth edition, http://www.novagen.com). The recombinant protein in the inclusion bodies was induced by IPTG in the strain Escherichia coli BL21 DE3 (Novagen). The purification of the recombinant protein from inclusion bodies was performed as described previously.10 The antisera were obtained by our modified immunisation methods.11 Antigen (600 µg) was injected into rabbits on days 1, 3 and 28. On the thirty-fifth day, the antisera were harvested from the carotid artery.
Protein extracts and Western blot analysis
Total protein extracts of mouse tissues were prepared as described previously.12 For the epididymal epithelial, luminal fluid and sperm protein extraction, the mouse epididymis was separated into three regions: caput (segments 1–5), corpus (segments 6 and 7) and cauda (segments 8–10), epididymal segments being numbered according to the classification of Johnston et al.13 The different epididymal regions were finely minced in phosphate-buffered saline (PBS; Gibco/BRL) supplemented with a protease inhibitor cocktail (Pierce, Rockford, IL, USA) and were incubated for 20 min at 37 °C to allow sperm release from the epididymal tubules. The supernatant containing spermatozoa and luminal contents was centrifuged at 500 g for 5 min; and the final supernatant was collected as luminal fluid protein extract and the sperm pellets were washed three times with cold PBS. The epididymal debris was also washed three times with cold PBS to remove the residual luminal fluid and spermatozoa. The tissue debris and sperm pellet were lysed with 1% (w/v) SDS supplemented with a protease inhibitor cocktail (Pierce) for 30 min and then centrifuged at 12 000 g for 15 min. The supernatants from each were collected as epithelial and sperm protein extracts, respectively.
Total protein extracts for each sample (12 µg) were separated on 12% (w/v) SDS–PAGE gels and semidry-blotted to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The polyclonal antiserum against mouse mHong1 recombinant protein was used as the primary antibody (dilution 1∶10 000). The second antibody was a goat horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (dilution 1∶20 000; CalBiochem, Darmstadt, Germany). The peroxidase activity was demonstrated with the ECL analysis system (Amersham Pharmacia Biotech).
Peptide N-glycosidase F treatment
Peptide N-glycosidase F treatment was performed by the procedure described previously.9 Briefly, 20 µg of total tissue protein extracts was treated and in the control sample no enzyme was added. The reaction products were then detected by Western blot analysis by using mHong1 polyclonal antisera.
Immunohistochemical staining of tissues
Tissue section preparation and immunohistochemical staining were performed as described previously.12 Primary and secondary antibodies were diluted in PBS containing 10% (v/v) normal goat serum. The 1∶200 diluted anti-mHong1 antiserum was applied to the tissues overnight at 4 °C and after the tissues were washed, 1∶200 diluted horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G was applied before incubation for 1 h at room temperature. As a negative control, serial sections were subjected to the same procedure, with normal rabbit serum replacing the primary antibody.
Indirect immunofluorescence staining of proteins associated with spermatozoa
The epididymides were separated into caput, corpus and cauda, as indicated above. The different segments were minced briefly in PBS and incubated at 37 °C for 15 min. The supernatants were carefully removed and centrifuged at 500 g for 5 min. The sperm pellets were washed twice with PBS and resuspended in 4% (w/v) paraformaldehyde at 37 °C for 10 min for fixation. After being washed with PBS, the spermatozoa were placed on polylysine-coated slides and air dried. The slides were blocked for 1 h at room temperature with 10% (v/v) goat serum in PBS. They were then incubated in polyclonal anti-mouse mHong1 serum (diluted 1∶400 in PBS containing 10% (v/v) goat serum) overnight at 4 °C, with pre-immune rabbit serum as the control. After three washes with PBST (PBS containing 0.2% (v/v) Tween-20), the corresponding second antibody was applied (FITC-conjugated anti-rabbit immunoglobulin G, 1∶600 diluted in PBS containing 10% (v/v) goat serum). The slides were washed three times with PBST and mounted in 80% (v/v) glycerol. Slides were examined in an Olympus BX-52 microscope.
High-salt solution treatment of spermatozoa
Mature C57 mice were killed and the epididymides were then removed and the caput, corpus and cauda segments were separated as indicated above. These segments were then minced in PBS at 37 °C and the sperm suspensions were filtered through cheesecloth (mesh size: 120 µm), divided into equal parts and centrifuged at 500 g for 10 min. The cells were washed once more in the same manner and then resuspended in either 0.5 ml PBS or 0.5 ml 0.5 mol l−1 NaCl and 0.0l mol l−1 sodium phosphate buffer at pH 7.2 (high-salt solution). The spermatozoa were incubated for 15 min at 37 °C and the suspensions centrifuged at 500 g for 10 min. The pelleted spermatozoa were then examined by indirect immunofluorescence.
Results
Cloning and sequence analysis of mHong1 cDNA
Two mouse homologous fragments (224 bp and 653 bp) were obtained after a BLAST search in the mouse genome by using the rat HongrES1 cDNA sequence (Figure 1a). On the basis of the mouse genomic sequence, four primers (FP1, RP1, FP2 and RP2) were designed and used to amplify the cDNA fragments by RT-PCR with mouse epididymal total RNA. The correct 224 bp and 653 bp fragments were amplified with primers FP1/RP1 and FP2/RP2, respectively and checked by automated sequencing. A 1524 bp cDNA fragment, which covered 224 bp and 657 bp fragments, was also amplified with primers FP1/RP2 (Figure 1b). On the basis of the sequence of this 1524 bp fragment, a gene-specific primer was designed, and the sequence toward the 3′ end was extended by 63 bp through 3′-RACE reactions. The full-length cDNA of mHong1 was 1587 bp. A polyadenylation signal (AATAAA) was located at 1548–1553 bp, 16 bp upstream of the poly (A) tail.
To identify the molecular size of the transcript of this mouse mHong1, total RNA isolated from the mouse epididymis and testis was analysed by Northern blotting. By using three different cDNA fragments (1–224 bp, 225–871 bp and 872–1524 bp) as probes, two transcripts of different size, one being about 1.6 kb and the other 3.7 kb, were obtained (Figure 1c). We performed the 5′-RACE to identify the extra sequence of the larger form of 3.7 kb, but no sequences were obtained (data not shown). In this study, we focused on the 1.6 kb transcript which is similar to HongrES1 in size.
The gene of mHong1 was located on mouse chromosome 12. It spanned 7336 bp and consisted of four introns and five exons (Figure 2b). As shown in Figure 2a, mHong1 contained a 1257 bp open reading frame encoding a protein with 418 amino acids.
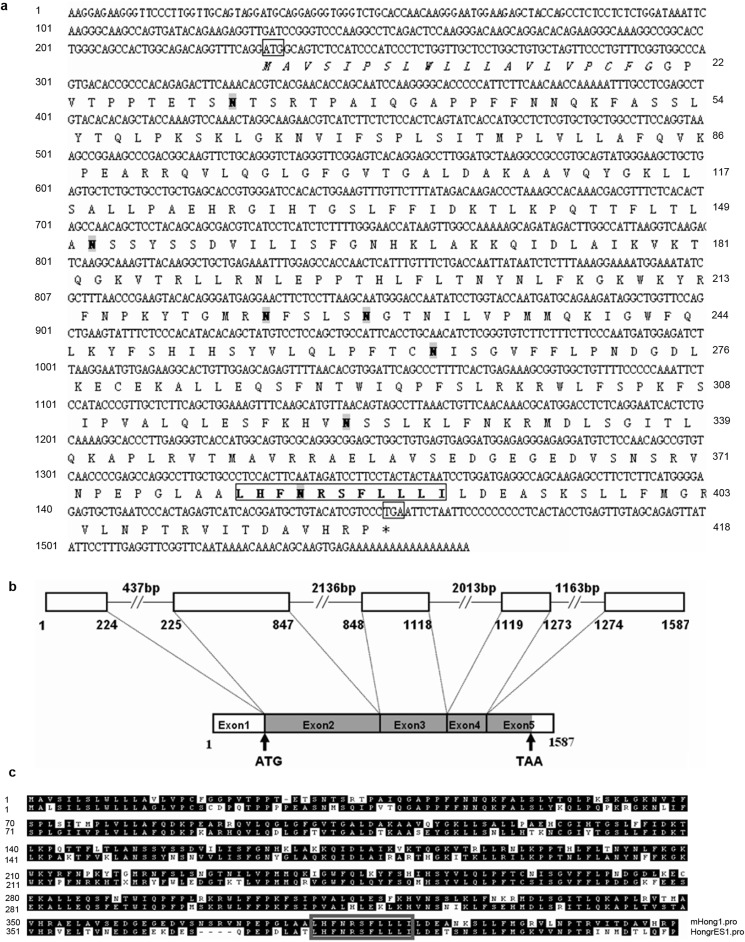
Figure 2.
The cDNA and deduced amino acid sequence analysis and the intron–exon structure of mHong1. (a) The mHong1 open reading frame contains 1257 bp coding for a 418-amino acid protein, and the initial and terminal codons are boxed. The protein contains a putative signal peptide (in italics) with a cleavage site between amino acids 20 and 21. The N-glycosylation (N31, 151, 223, 228, 263, 322 and 383) sites are shadowed grey. The serpin motif from 380th to 390th amino acid is in bold and boxed. (b) The mHong1 gene diagram shows the relative lengths of exons 1–5 and intervening introns. The shadowed grey box represents the open reading frame of mHong1 mRNA. (c) Amino-acid sequence alignment of mouse mHong1 and rat HongrES1. Dashes show alignment gaps. The conserved serpin motif residues are indicated by grey box.
The N-terminal 20 amino acids may form a signal peptide, as predicted with the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/). Cleavage of this peptide would lead to a mature protein of 398 amino acids with an estimated molecular mass of 44.7 kDa and isoelectric point of 10.0. Seven potential N-glycosylation sites were predicted at N31, 151, 223, 228, 263, 322 and 383. Seven other sites of potential post-translational modification are present in this sequence: four protein kinase C phosphorylation sites located at T32SR, T139LK, S317FK and S324LK; and three N-myristoylation sites located at G96LGFGV, G103ALDAK and G127IHTGS. A serpin signature from the 380th to 390th amino-acid residues was found by the software at the site: http://myhits.isb-sib.ch/cgi-bin/motif_scan (Figure 2c). A BLAST search in the GenBank protein database shows that mHong1 protein shares 71% similarity to rat HongrES1.
mHong1 mRNA is predominantly expressed in the epididymis and upregulated by androgen but not other testicular factors
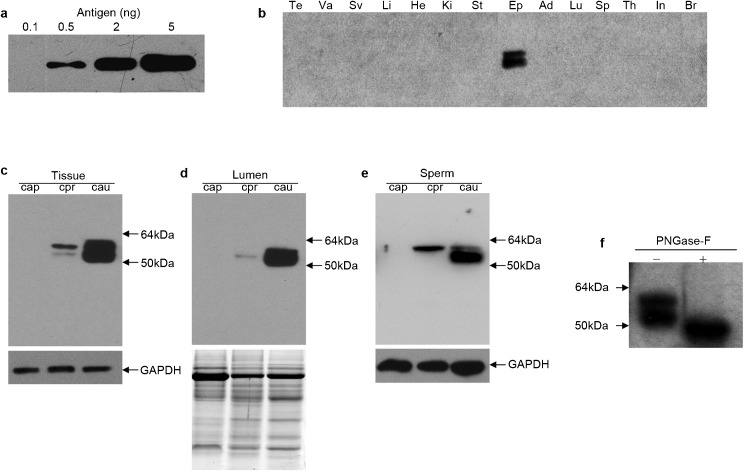
To determine the tissue expression pattern of mHong1, we performed Northern blot hybridisation on the RNA samples prepared from 14 different tissues of adult male mice. As shown in Figure 3a, mHong1 mRNA was predominantly expressed in the epididymis, and a weak signal in the vas deferens could also be detected. No signal was observed in other tissues. mHong1 mRNA was highly regionalized and predominantly expressed in the corpus and cauda epididymidis without expression in the caput region.
Figure 3.
Tissue distribution, androgen-dependent and testicular factor-independent expression of mHong1 mRNA by Northern blot analysis. (a) Total RNAs (15 µg per lane) from various tissues were used. The mHong1 mRNA is highly expressed in the mouse epididymis, especially in the corpus and cauda, and weakly expressed in vas deferens but not in the other tissues. Cap, caput; Cor, Corpus; Cau, Cauda; Te, testis; Ep, epididymis; Va, vas deferens; Sv, seminal vesicle; Li, liver; He, heart; Ki, kidney; St, stomach; Br, brain; In, intestine; Th, thymus; Sp, spleen; Lu, lung; Ad, adrenal. (b) Northern blot analysis of adult mouse epididymal RNAs from pre-castration (d0) and bilateral castration for 1,3, 5 and 7 days (d1, d3, d5 and d7) as well as for 1, 3, 5 and 7 days after the initial injection of testosterone propionate to the 7-day castrated rats (d7+1, d7+3, d7+5 and d7+7). The total RNAs were pooled from six animals at each time point. (c) The relative expression levels of mHong1 mRNA (hybridisation density of mHong1 mRNA/18s ribosomal RNA) in the mouse epididymis and the serum testosterone level (expressed in nmol l−1) during androgen manipulation. (d) Northern blot analysis of mHong1 mRNA and 18s rRNA in the epididymis of efferent duct-ligated mice and control mice. The left side of each rat epididymis (1L, 2L, 3L, 4L) was ligated, and the other side (1R, 2R, 3R, 4R) served as the control. The RNAs were pooled with four animals per group. (e) The relative expression levels of transcripts (hybridisation density of mHong1 mRNA/18s ribosomal RNA) in the mouse epididymis.
The effects of androgen manipulation on mHong1 mRNA expression were analysed by Northern blot analysis. As shown in Figure 3b and 3c, mHong1 mRNA in the epididymis was dramatically decreased in abundance concomitant with the drastic decrease in serum testosterone after castration. After the administration of testosterone to mice that had been castrated for 7 days, mHong1 mRNA gradually returned to nearly normal levels in parallel with serum testosterone restoration.
Testicular factors other than testosterone from the testis play an important role in regulating epididymal gene expression.14, 15 Fourteen days after unilateral efferent duct ligation, mHong1 mRNA levels in the ligated and unligated epididymis were almost identical to each other (Figure 3d and 3e).
The native status of mHong1 protein in the epididymis
To characterize mHong1 at the protein level, we generated a rabbit polyclonal antiserum against the recombinant C-terminal peptide of mHong1. The sensitivity and specificity of the antiserum was examined by Western blot analysis. As shown in Figure 4a, as little as 0.5-ng C-terminal antigen could be readily detected by the antiserum at a 1∶20 000 dilution. Two proteins were detected in the extract from the epididymis but not in the testis or other tissues (Figure 4b), which is consistent with the tissue distribution pattern of the mHong1 mRNA. The sizes of both proteins (molecular mass: ≈54 kDa and 57 kDa) were larger than the deduced mature mHong1 protein (molecular mass: 47.4 kDa). After deglycosylation of the tissue protein extracts by N-glycosidase, only one band of about 47 kDa was detected by Western blot analysis (Figure 4f), which is identical to the deduced size.
Figure 4.
Western blot analysis of mHong1 native protein in tissues. (a) Rabbit polyclonal antisera were raised against mHong1 C-terminal peptide (antigen) and the sensitivity of the antibody towards antigen was verified by Western blotting: 0.1, 0.5, 2 and 5 ng of antigen peptide were loaded. (b) Protein extracts (12 µg per lane) from various tissues were analysed. Te, testis; Va, vas deferens; Sv, seminal vesicle; Li, liver; He, heart; Ki, kidney; St, stomach; Ep, epididymis; Ad, adrenal; Lu, lung; Sp, spleen; Th, thymus; In, intestine; Br, brain. (c–e) Western blot analysis of mHong1 in epithelium (c), lumen (d) and sperm (e) protein extracts from caput (Cap), corpus (Cor) and cauda (Cau) epididymidis. GAPDH was used as an internal control in (c) and (e). The same amount of protein was separated by electrophoresis and stained by Coomassie blue to demonstrate equal loading in (d). (f) The change of molecular masses of mHong1 protein in total tissue protein of cauda epididymidis before (−) and after (+) deglycosylation by peptide N-glycosidase F (PNGase-F).
Regional, cell-specific expression and secretory activity of mHong1 in the epididymis
As shown in Figures 4c and 5, mHong1 was detected in the corpus and cauda regions of the mouse epididymis but not in the caput region. In Figure 5a, it is seen that there is a gradually increasing immunoreactivity in the epithelium and lumen from the proximal corpus to the distal cauda region. The magnified images in Figure 5b show that no signal was found in the caput region (Figure 5b), and the immunoreactive signal in the epithelium and lumen was strong in the cauda region (Figure 5b, b8 and b9) and moderate in the corpus region (Figure 5b, b4-b7). It is noteworthy that not all the epithelial cells expressed mHong1, and the epithelium presented a checker-board protein staining pattern.
Figure 5.
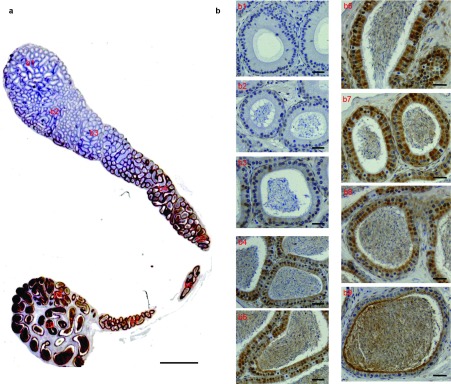
The region-specific localisation of mHong1 in the mouse epididymis. (a) The expression pattern of mHong1 in the whole mouse epididymis. Bars=2 mm. (b) The magnified photographs for individual fields of (a): initial segment (b1), caput (b2), distal caput (b3), proximal corpus (b4), corpus (b5, b6), distal corpus (b7), proximal cauda (b8) and distal cauda (b9). Bars=50 µm.
To examine whether mHong1 is a secretory protein, protein extracts of the epididymal epithelial cells and luminal fluid from the different regions were analysed by Western blot analysis. The mHong1 protein was present in both the epithelium and lumen of the corpus and cauda regions (Figure 4c and d).
Immunolocalisation of mHong1 on spermatozoa
Indirect immunofluorescence staining showed that mHong1 was concentrated over the equatorial segment region of spermatozoa (Figure 6b). In the cauda, most spermatozoa could bind mHong1, whereas only a small percentage of spermatozoa in the corpus showed a weak fluorescence; caput spermatozoa did not show fluorescent staining (Figure 6a).
Figure 6.
The localisation of mHong1 protein on spermatozoa by indirect immunofluorescence assays. (a) Immunolocalisation of mHong1 (FITC-labelled) on spermatozoa isolated from different epididymal regions. Cauda spermatozoa detected by pre-immune serum as negative control. Bars=10 µm. (b) mHong1 localisation on spermatozoa from the cauda epididymidis. (b1) Phase contrast view of spermatozoa in (b2). (b2) The immunofluorescence of mHong1 (FITC-labelled). (b3) Sperm nucleus detected by propidium iodide (PI). (b4) Merged micrograph of (b1) and (b2). (b5) Merged micrograph of (b1), (b2) and (b3). (b6) Phase contrast view of spermatozoa in (b7). (b7) Cauda spermatozoa detected by pre-immune serum as negative control. (b8) Sperm nucleus detected by propidium iodide (PI). (b9) Merged micrograph of (b6) and (b7). (b10) Merged micrograph of (b6), (b7) and (b8). Bars=50 µm. (c) mHong1 can be removed from cauda spermatozoa by washing with high-salt solution (HSS) but not phosphate-buffered saline (PBS). Bars=10 µm.
mHong1 can be removed from the sperm surface by high-salt treatment
To demonstrate how mHong1 interacts with the sperm surface, cauda spermatozoa were treated with high-salt solution or PBS and were analysed by indirect immunofluorescence staining. As shown in Figure 6c, after high-salt solution treatment, no mHong1 immunostaining signal could be detected on the sperm surface, but after PBS treatment, the immunostaining signal was not decreased from that of the untreated cells.
Discussion
In the present study, we identified and characterized the mHong1 gene in the mouse epididymis at both the mRNA and protein levels. From the data on the genomic structure, the amino-acid sequence and its tissue distribution, mouse mHong1 can be certainly identified as the homologue of rat HongrES1.
During our study, a predicted gene, Gm46, located in the Serpin cluster of the chromosome 12, was annotated by the Human and Vertebrate Analysis and Annotation project. This gene is shown to be orthologous to HongrES1 and its sequence is identical to that of mHong1. However, no information about its expression, distribution, regulation and function was provided in this gene term.
The mHong1 gene is also recognized as a new member of serpin family owing to its typical serpin domain. But, as with HongrES1, whether mHong1 is functionally related to the epididymal fluid proteinase inhibitors must remains a matter for speculation, since the amino acids in the hinge region of the reactive centre loop of mHong1 were large residues (Val, Asp, Glu), which is different from the small residues of traditional inhibitory serpins.16
Our results suggest that mHong1 mRNA expression is regulated by testosterone in vivo. Androgenic regulation on gene expression is mediated by androgen receptor, a nuclear receptor that belongs to the ligand-inducible transcription factor superfamily. The binding of the androgen receptor to specific cis-acting DNA regulatory elements named the androgen response element results in the modulation of the target gene transcription. We searched the transcription factor binding sites in the proximal promoter region (1000 bp-long stretch of 5′ upstream region) with Genomatix software and found three stretches resembling the androgen response element consensus sequence GGWACANNNTGTTCT in either orientation: -648, CATGCTGACCTTGTTCTGT; -654, AAGGTACATGCTGACCTTG; -784, CAGGATGTCTCTGTTCTTG. Therefore, mHong1 gene expression may be directly regulated by androgens at the transcriptional level, but its expression is independent of the presence of testicular factors other than testosterone (Figure 3b–e).
Many genes in the epididymis exhibit highly regionalized expression that is thought to be critical for epididymal function.17 In our work, mHong1 showed region-specific expression pattern, but its expression pattern was somewhat different from that of HongrES1, whose mRNA and protein were both exclusively expressed in the cauda region. Any biological significance for the observed species differences in HongrES1/mHong1 is unknown. The differential expression patterns may be indicative of different roles of these two proteins in sperm maturation.
About 150–200 proteins are estimated to be secreted by the epididymal epithelium,18 but few of them have been shown to function in sperm maturation. The principal characteristic of these secretory proteins is high polymorphism, both in molecular weight and isoelectric point. Most of them are secreted in the proximal part of the epididymis. In the boar, the protein secretion in the caput, corpus and cauda epididymis represents 83%, 16% and 1% respectively of the overall secretion of the epididymis.19 In our work, mHong1 protein was identified as a corpus- and cauda-specific secretory protein and especially rich in cauda lumen (Figure 4c and d). It is also a polymorphous protein as judged by the appearance of a 57/54 kDa doublet band (Figure 4b) in the epididymal protein extracts detected by Western blots because of the high glycosylation.
Although HongrES1 protein shares 71% similarity to mHong1, it is quite different from mHong1 in post-translational modifications. Compared with the deduced band, only one higher MW band of HongrES1 was detected in rat epididymal protein extracts by Western blots.6 After deglycosylation, an about 2 kDa shift in molecular mass of the HongrES1 band was detected.6 It was found that there were seven N-glycosylation sites in the mHong1 amino acid sequence, but only three for rat HongrES1. This is accounted for by the different extents of glycosylation of mHong1 and HongrES1. While the role of the glycosylated isoforms of mHong1 remains unknown, it is conceivable that glycosylation is related to mHong1 protein activity, such as folding, secretion, transport and interaction with the sperm surface.
It is well established that the sperm plasma membrane undergoes substantial remodelling to induce sperm maturation during epididymal transit.20 Some of these surface changes result from interactions with proteins produced by the epididymis.21, 22 In the present study, we observed that mHong1 can be deposited onto the sperm equatorial segment (Figure 6a and b). The sperm-binding pattern of mHong1 is significantly different from that of HongrES1 in rat and guinea pig spermatozoa,6, 7 so they probably have different functions in reproduction. In the rat, HongrES1 covered the whole sperm head region; and as a decapacitation factor, it is thought to enable spermatozoa to acquire capacitation at the right time and right place. In the guinea pig, HongrES1 is localized to the anterior sperm acrosomal region. During capacitation, HongrES1 is lost gradually and is removed completely after the acrosome reaction. Therefore, removal of HongrES1 during capacitation may be required for guinea pig sperm activation. It is well documented that the equatorial segment is of considerable functional importance for fertilisation;23, 24 therefore, mHong1 may be involved in the fertilisation process. Further experiments will be needed to test this hypothesis.
The mHong1 protein can be removed from sperm membrane by high ionic strength and therefore can be classed as an extrinsic membrane protein. The requirement of elevated ionic strength for the removal of the mHong1 protein is also consistent with the idea that its binding to the sperm surface is receptor-mediated. At this time, nothing is known about what the receptors of mHong1 on sperm are. It is noteworthy that mHong1 is a hydrophilic glycoprotein, being rich in positively-charged amino acids and attached glycans, so we hypothesize that hydrophilic interactions with negatively-charged sperm membrane receptors are responsible for the attachment of mHong1 to the sperm surface. Further study will be needed to check this hypothesis and search for the receptors.
Taken together, our results allow us to identify mHong1 as the homolog of HongrES1, and our work paves the way for establishing animal models to elucidate the precise functions of HongrES1 and mHong1.
Author contributions
SGH and HD designed and performed experiments, analysed data and wrote the paper; GXY provided great help in immunohistochemistry assays; YLZ designed experiments and wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
We thank Dr Qiang Liu for giving some advice and polishing the writing and thank Dr Yu-Chuan Zhou for fruitful discussions. We are also grateful to Ai-Hua Liu for technical assistance in immunohistochemistry assay. This work was supported by grants from the National Natural Science Foundation of China (No. 30930053) and the Chinese Academy of Sciences Knowledge Innovation Program (No. KSCX2-EW-R-07).
The authors declare that they have no competing financial interests.
References
- Rodriguez CM, Kirby JL, Hinton BT.The development of the epididymisIn: Robaire B, Hinton BT, editors.The Epididymis: From Molecules to Clinical Practice New York; Kluwer Academic/Plenum Publishers; 2002p251 [Google Scholar]
- Roberts KP, Wamstad JA, Ensrud KM, Hamilton DW. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol Reprod. 2003;69:572–81. doi: 10.1095/biolreprod.102.013771. [DOI] [PubMed] [Google Scholar]
- Zhou CX, Zhang YL, Xiao L, Zheng M, Leung KM, et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat Cell Biol. 2004;6:458–64. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Li JY, Li F, Wang HY, Shi GX. Human ribonuclease 9, a member of ribonuclease A superfamily, specifically expressed in epididymis, is a novel sperm-binding protein. Asian J Androl. 2009;11:240–51. doi: 10.1038/aja.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZH, Liu Q, Shang Q, Zheng M, Yang J, et al. Identification and characterization of a new member of serpin family—HongrES1 in rat epididymis. Cell Res. 2002;12:407–10. doi: 10.1038/sj.cr.7290143. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zheng M, Shi Q, Zhang L, Zhen W, et al. An epididymis-specific secretory protein HongrES1 critically regulates sperm capacitation and male fertility. PLoS ONE. 2008;3:e4106. doi: 10.1371/journal.pone.0004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Zhou Y, Chen WY, Zheng M, Yu J, et al. HongrES1, a cauda epididymis-specific protein, is involved in capacitation of guinea pig sperm. Mol Reprod Dev. 2009;76:984–93. doi: 10.1002/mrd.21063. [DOI] [PubMed] [Google Scholar]
- Li P, Chan HC, He B, So SC, Chung YW, et al. An antimicrobial peptide gene found in the male reproductive system of rats. Science. 2001;291:1783–5. doi: 10.1126/science.1056545. [DOI] [PubMed] [Google Scholar]
- Zhu CF, Liu Q, Zhang L, Yuan HX, Zhen W, et al. RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol Reprod. 2007;76:63–73. doi: 10.1095/biolreprod.106.054635. [DOI] [PubMed] [Google Scholar]
- Hu ZH, Li B, Liu Q, Zhang YL. Expression, purification of the recombinant protein fragment of the monkey epididymis expressed gene ESc-615 and preparation of polyclonal antisera to it. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2002;34:753–6. [PubMed] [Google Scholar]
- Hu YX, Guo JY, Shen L, Chen Y, Zhang ZC, et al. Get effective polyclonal antisera in one month. Cell Res. 2002;12:157–60. doi: 10.1038/sj.cr.7290122. [DOI] [PubMed] [Google Scholar]
- Xiao LQ, Liu AH, Zhang YL. An effective method for raising antisera against beta-defensins: double-copy protein expression of mBin1b in E. coli. Acta Biochim Biophys Sin (Shanghai) 2004;36:571–6. doi: 10.1093/abbs/36.8.571. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- Cornwall GA, Collis R, Xiao Q, Hsia N, Hann SR. B-Myc, a proximal caput epididymal protein, is dependent on androgens and testicular factors for expression. Biol Reprod. 2001;64:1600–7. doi: 10.1095/biolreprod64.6.1600. [DOI] [PubMed] [Google Scholar]
- Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–64. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Hann SR. Specialized gene expression in the epididymis. J Androl. 1995;16:379–83. [PubMed] [Google Scholar]
- Dacheux JL, Druart X, Fouchecourt S, Syntin P, Gatti JL, et al. Role of epididymal secretory proteins in sperm maturation with particular reference to the boar. J Reprod Fertil Suppl. 1998;53:99–107. [PubMed] [Google Scholar]
- Dacheux JL, Dacheux F.Protein secretion in the epididymisIn: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice New York; Kluwer Academic/Plenum Publishers;2002pp151–68. [Google Scholar]
- Yamaguchi R, Yamagata K, Hasuwa H, Inano E, Ikawa M, et al. Cd52, known as a major maturation-associated sperm membrane antigen secreted from the epididymis, is not required for fertilization in the mouse. Genes Cells. 2008;13:851–61. doi: 10.1111/j.1365-2443.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Marengo SR. Maturing the sperm: unique mechanisms for modifying integral proteins in the sperm plasma membrane. Anim Reprod Sci. 2008;105:52–63. doi: 10.1016/j.anireprosci.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–91. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Wolkowicz MJ, Digilio L, Klotz K, Shetty J, Flickinger CJ, et al. Equatorial segment protein (ESP) is a human alloantigen involved in sperm–egg binding and fusion. J Androl. 2008;29:272–82. doi: 10.2164/jandrol.106.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowicz MJ, Shetty J, Westbrook A, Klotz K, Jayes F, et al. Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol Reprod. 2003;69:735–45. doi: 10.1095/biolreprod.103.016675. [DOI] [PubMed] [Google Scholar]