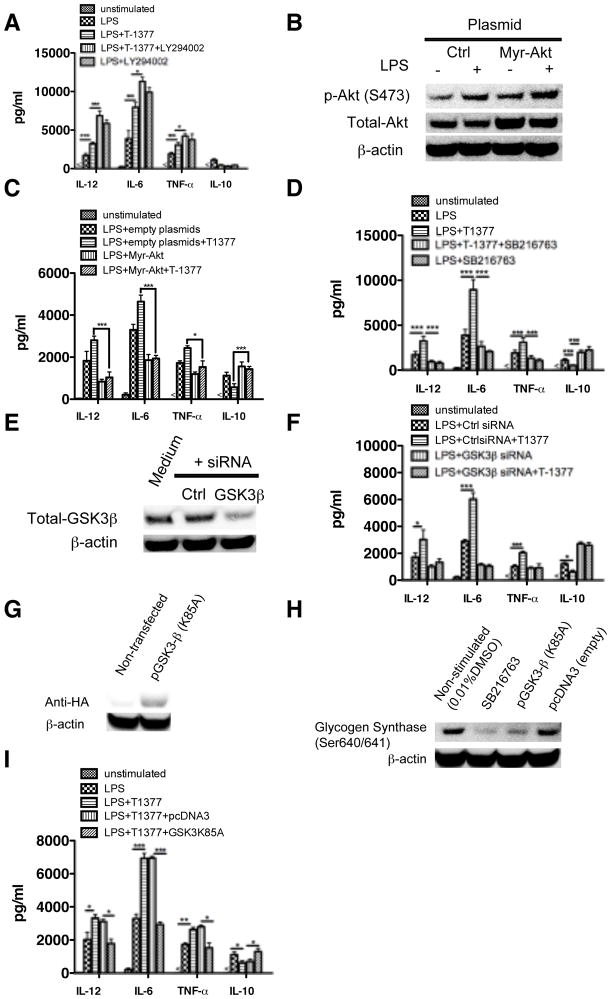
Figure 4. The ability of JAK3 inhibition to regulate TLR4 mediated inflammatory cytokine production is dependent on the activity of PI3K and GSK3β.
The levels of IL-12, TNF-α, IL-6, and IL-10 were assayed by ELISA in LPS stimulated human monocytes. The regulatory effect of JAK3 inhibition was (A) significantly enhanced by PI3K inhibition (LY294002) and (B, C) abrogated by constitutive expression of Akt. Inhibition of GSK3 mediated by pharmacological inhibitor (D) or specific siRNA (E, F) attenuated the regulatory ability of JAK3 on LPS-mediated inflammatory cytokine production in human monocytes. (G) HA expression levels were detected by Western Blot 48 h post-transfection in nontransfected monocytes and monocytes transfected to confirm the transfection efficiency of kinase dead (K85A) plasmid encoding GSK3β. (H) monocytes transfected with kinase dead (K85A) plasmid exhibited a loss in the phosphorylation levels of the GSK3 specific substrate glycogen synthase (GS) (S640/641). (I) As compared with monocytes transfected with empty vector control, the kinase dead (K85A) GSK3β mutant abrogated the ability of JAK3 inhibition to increase the production of TNF-α, IL-12, IL-6, and decrease the IL-10 level in LPS stimulated cells. *, **, and *** indicates statistically significant at P<0.05, P<0.01 and P<0.001, respectively. Data represent the arithmetic mean ± S.D. of three biological replicates.