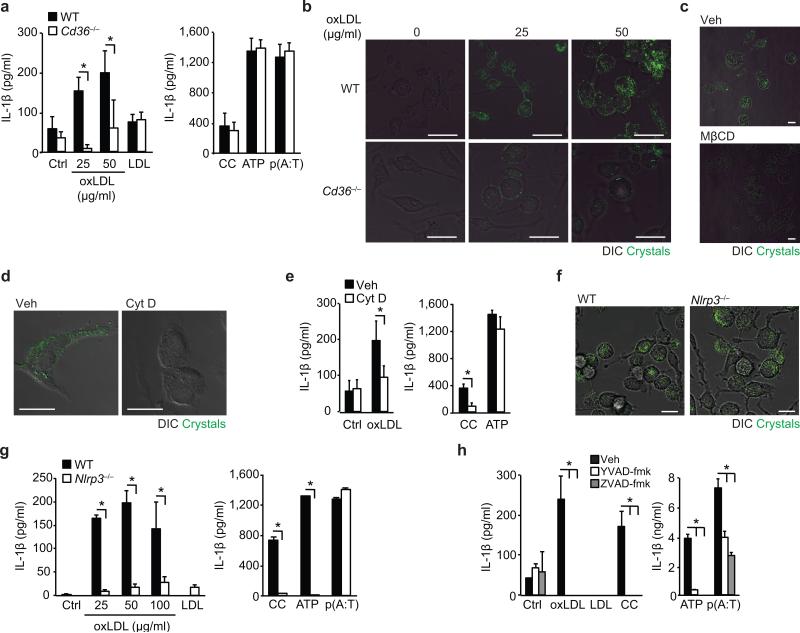
Figure 1. CD36-mediated uptake of oxLDL generates intracellular crystals and activates the NLRP3 inflammasome.
(a) IL-1β ELISA of supernatants from LPS-primed wild-type (WT) or CD36-deficient (Cd36-/-) BMDM incubated for 24 h with PBS vehicle (Ctrl), oxLDL or unmodified LDL (left panel) alongside the following inflammasome activators (right panel), cholesterol crystals (CC, 1 mg/mL), ATP (5 μM, 1 h) and poly(dA:dT) [p(A:T), 100 ng/well, 6 h]. (b) Confocal reflection microscopy of WT or Cd36-/- peritoneal macrophages treated for 24 h with oxLDL as indicated. (c) Confocal reflection microscopy of peritoneal macrophages treated with oxLDL (50 μg/mL, 24 h) and subsequently treated with methyl-β-cyclodextrin (MβCD, 10 μM, 1 h) or vehicle (Veh). (d) Confocal reflection microscopy of oxLDL-treated BMDM (50 μg/mL, 24 h) following pre-treatment with cytochalasin D (Cyt D (1 μM, 1 h) or vehicle control (Veh). (e) IL-1β ELISA of supernatants from LPS-primed BMDM treated for 24 h with PBS vehicle (Ctrl), oxLDL (50 μg/mL) or cholesterol crystals and ATP as before. (f) Confocal reflection microscopy of WT or NLRP3-deficient (Nlrp3-/-) BMDM treated with oxLDL (50 μg/mL, 24 h). (g) IL-1β ELISA of supernatants from LPS-primed WT or Nlrp3-/- BMDM treated as in a). (h) IL-1β ELISA of supernatants from LPS-primed BMDM treated with the indicated inflammasome activators following 1 h pre-treatment with ZVAD-fmk (pan-caspase inhibitor, 20 μM) or YVAD-fmk (caspase-1 inhibitor, 20 μM). Data in a, e, g and h are mean ± s.d. of triplicate samples within a single experiment and are representative of three independent experiments. Images in b, c,d and f are representative of 3 independent experiments. Scale bar = 10 μm. *P<0.05.